Abstract
Background
Insulin might be associated with changes in infant gastrointestinal microbiota. The objective of this randomized controlled trial was to assess the efficacy of two doses of recombinant human(rh) enteral insulin administration compared to placebo in intestinal microbiota.
Methods
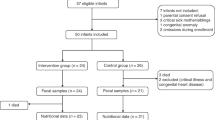
19 preterm patients were recruited at the NICU of La Paz University Hospital (Madrid, Spain). Subjects received 2000 µIU of rh enteral insulin/ml(n = 8), 400 µIU of rh enteral insulin/ml(n = 6) or placebo(n = 5) for 28 days administered once per day. Extracted DNA from fecal samples collected at the beginning and end of treatment were analyzed. The 16S rRNA V4 region was amplified and sequenced in a Miseq(Illumina®) sequencer using 2 × 250 bp paired end. Resulting reads were filtered and analyzed using Qiime2 software. Metabolic activity was assessed by GC.
Results
Gestational age and birth weight did not differ between groups. At the phylum level, both insulin treated groups increased the relative abundance of Bacillota, while Pseudomonadota decreased. No change was observed in infants receiving placebo. At the genus level, insulin at both doses showed enriching effects on Clostridium. We found a significant increase in concentrations of fecal propionate in both rh insulin treated groups.
Conclusion
Rh insulin may modify neonatal intestinal microbiota and SCFAs in preterm infants.
Impact statement
-
Decrease of Pseudomonadota (former Proteobacteria phylum) and increase of Bacillota (former Firmicutes phylum) obtained in this study are the changes observed previously in low-risk infants for NEC.
-
The administration of recombinant enteral insulin may modify the microbiota of preterm new-borns and SCFAs.
-
Modulation of the microbiota may be a mechanism whereby insulin contributes to neonatal intestinal maturation and/or protection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Rao, C. et al. Multi-kingdom ecological drivers of microbiota assembly in preterm infants. Nature 591, 633–638 (2021).
Lynch, S. V. & Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379 (2016).
Honda, K. & Littman, D. R. The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84 (2016).
Mark A.U. et al. A comparison of two probiotic strains of bifidobacteria in preterm infants. 163:1585–1591.e9 (2013).
Escribano, E. et al. Increased incidence of necrotizing enterocolitis associated with routine administration of InfloranTM in extremely preterm infants. Benef. Microbes 9, 683–690 (2018).
Watkins, C. et al. Dose-interval study of a dual probiotic in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 104, F159–F164 (2019).
Xiao, L. et al. Effect of probiotics on digestibility and immunity in infants. Medicine 96, e5953 (2017).
Chang, H. Y. et al. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: An updated meta-analysis. PLoS One 12, 1–14 (2017).
Wu, B. B., Yang, Y., Xu, X. & Wang, W. P. Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J. Pediatr. 12, 177–182 (2016).
Plummer, E. L. et al. Gut microbiota of preterm infants supplemented with probiotics: sub-study of the ProPrems trial. BMC Microbiol 18, 1–8 (2018).
Marzotto, M. et al. Lactobacillus paracasei A survives gastrointestinal passage and affects the fecal microbiota of healthy infants. Res. Microbiol. 157, 857–866 (2006).
Fatheree, N. Y. et al. Lactobacillus reuteri for infants with colic: a double-blind, placebo-controlled, randomized clinical trial. J. Pediatr. 191, 170–178.e2 (2017).
Rougé, C. et al. Oral supplementation with probiotics in very-low-birth-weight preterm. Am. J. Clin. Nutr. 89, 1828–1835 (2009).
Nation, M. L. et al. Impact of Lactobacillus reuteri colonization on gut microbiota, inflammation, and crying time in infant colic. Sci. Rep. 7, 1–8 (2017).
Rinke, C. et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature 499, 431–437 (2013).
Soergel, D. A. W., Dey, N., Knight, R. & Brenner, S. E. Selection of primers for optimal taxonomic classification of environmental 16S rRNA gene sequences. ISME J. [Internet] 6, 1440–1444 (2012).
Caporaso, J. G. et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624 (2012).
Rauf, A. et al. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): an updated review. Crit. Rev. Food Sci. Nutr. 62, 6034–6054 (2022).
Rios-Covian, D. et al. An overview on fecal branched short-chain fatty acids along human life and as related with body mass index: associated dietary and anthropometric factors. Front. Microbiol. 11, 1–9 (2020).
Den Besten, G. et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340 (2013).
Ríos-Covián, D. et al. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 7, 1–9 (2016).
Shehadeh, N., Sukhotnik, I. & Shamir, R. Gastrointestinal tract as a target organ for orally administered insulin. J. Pediatr. Gastroenterol. Nutr. 43, 276–281 (2006).
Shulman, R. J. Oral insulin increases small intestinal mass and disaccharidase activity in the newborn miniature pig. Pediatr. Res. 28, 171–175 (1990).
Mank, E. et al. Thermoultrasonication, ultraviolet-C irradiation, and high-pressure processing: novel techniques to preserve insulin in donor human milk. Clin. Nutr. 40, 5655–5658 (2021).
Mank, E. et al. Efficacy and safety of enteral recombinant human insulin in preterm infants: a randomized clinical trial. JAMA Pediatr. 176, 452–460 (2022).
Shulman, R. J. Effect of enteral administration of insulin on intestinal development and feeding tolerance in preterm infants: a pilot study. Arch. Dis. Child Fetal Neonatal Ed. 86, 131–133 (2002).
Lemas, D. J. Alterations in human milk leptin and insulin are associated with early changes in the infant intestinal microbiome. Am. J. Clin. Nutr. 103, 1291–1300 (2016).
Bolyen, E. et al. Reproducible, interactive, scalable, and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Zimmerman, T. W., Reinprecht, J. T. & Binder, H. J. Peptide binding to intestinal epithelium: distinct sites for insulin, EGF and VIP. Peptides 6, 229–235 (1985).
Slebodzinski, A. B., Nowak, J., Gawecka, H. & Sechman, A. Thyroid hormones and insulin in milk: a comparative study. Endocrinol. Exp. 20, 247–255 (1986).
Yong, J. H. et al. Dietary insulin affects leucine aminopeptidase, growth hormone, insulin-like growth factor I, and insulin receptors in the intestinal mucosa of neonatal pigs. Biol. Neonate 89, 265–273 (2006).
Buts, J.-P., de Keyser, N., Kolanowski, J. & Van Hoof, F. Hormonal regulation of the rat small intestine: responsiveness of villus and crypt cells to insulin during the suckling period and unresponsiveness after weaning. Pediatr. Res. 27, 161–164 (1990).
Forgue-Lafitte, M. E., Marescot, M. R., Chamblier, M. C. & Rosselin, G. Evidence for the presence of insulin binding sites in isolated rat intestinal epithelial cells. Diabetologia 19, 373–378 (1980).
Sodoyez-Goffaux, F., Sodoyez, J. C. & De Vos, C. J. Insulin receptors in the gastrointestinal tract of the rat fetus: quantitative autoradiographic studies. Diabetologia 28, 45–50 (1985).
Georgiev, I. P., Georgieva, T. M., Pfaffl, M., Hammon, H. M. & Blum, J. W. Insulin-like growth factor and insulin receptors in intestinal mucosa of neonatal calves. J. Endocrinol. 176, 121–132 (2003).
Lobie, P. E., Breipohl, W. & Waters, M. J. Growth hormone receptor expression in the rat gastrointestinal tract*. Endocrinology 126, 299–306 (1990).
Young, G. P. et al. Insulin-like growth factors and the developing and mature rat small intestine: receptors and biological actions. Digestion 46, 240–252 (1990).
Hammon, H. M. & Blum, J. W. Feeding different amounts of colostrum or only milk replacer modify receptors of intestinal insulin-like growth factors and insulin in neonatal calves. Domest. Anim. Endocrinol. 22, 155–168, https://www.sciencedirect.com/science/article/pii/S0739724002001224 (2002).
Xu, R. J. et al. Effects of oral IGF-I or IGF-II on digestive organ growth in newborn piglets. Biol. Neonate 66, 280–287 (1994). (1994).
Houle, V. M., Schroeder, E. A., Odle, J. & Donovan, S. M. Small intestinal disaccharidase activity and ileal villus height are increased in piglets consuming formula containing recombinant human insulin-like growth factor-I. Pediatr. Res. 42, 78–86 (1997).
Burrin, D. G., Wester, T. J., Davis, T. A., Amick, S. & Heath, J. P. Orally administered IGF-I increases intestinal mucosal growth in formula-fed neonatal pigs. Am. J. Physiol. 270, R1085–R1091 (1996).
Buts, J. P., De Keyser, N., Sokal, E. M. & Marandi, S. Oral insulin is biologically active on rat immature enterocytes. J. Pediatr. Gastroenterol. Nutr. 25, 230–232 (1997).
Boudry, G. et al. The relationship between breast milk components and the infant gut microbiota. Front. Nutr. 8, 1–21 (2021).
Al-Majali, A. M. et al. Insulin modulates intestinal response of suckling mice to the Escherichia coli heat-stable enterotoxin. Adv. Exp. Med. Biol. 473, 113–123 (1999).
Pammi, M. et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5, 1–15 (2017).
Warner, B. B. et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 387, 1928–1936 (2016).
Claud, E. C. et al. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome 1, 20 (2013).
Mai, V. et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE 6, e20647 (2011).
Wang, Y. et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 3, 944–954 (2009).
Lu, P. & Hackam, D. J. Inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology 21, 81–93 (2015).
Alsharairi, N. A. Therapeutic potential of gut microbiota and its metabolite short-chain fatty acids in neonatal necrotizing enterocolitis. Life 13, 561 (2023).
Pourcyrous, M., Nolan, V. G., Goodwin, A., Davis, S. L. & Buddington, R. K. Fecal short-chain fatty acids of very-low-birth-weight preterm infants fed expressed breast milk or formula. J. Pediatr. Gastroenterol. Nutr. 59, 725–731 (2014).
Acknowledgements
We wish to particularly acknowledge and thank the study participants and their families for their contribution to the study and the Biobank IdiPAZ (PT20/00004) integrated in the Spanish National Biobanks Network for their collaboration. The trial was previously funded by Nutrinia Ltd. (Ramat Gan, Israel), which acted as sponsor of the study at that time (currently, Elgan Pharma (Nazareth, Israel) acts as the sponsor). The authors had final responsibility for the design and conduct of this study. The sponsor had no role in the collection, management, analysis, and interpretation of the data. The sponsor was allowed to review and comment on the manuscript. The final version and decision to publish was made by the authors without contractual limits.
Author information
Authors and Affiliations
Contributions
B.M.-S.: Methodology, Validation, Formal Analysis, Investigation, Resources, Data Curation, Writing and Original Draft, Project Administration. F.L.P: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing, and Original Draft. E.E.: Methodology, Investigation, Data curation, Writing and Review and Editing, Supervision. M.C.L.: Conceptualization, Methodology, Investigation, Data curation, Writing and Review and Editing, Supervision. M.T.M..: Methodology, Investigation. R.A.: Methodology, Investigation. S.A.: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing, and Original Draft. M.G.: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing, and Original Draft. J.M.: Methodology, Validation, Formal analysis, Investigation, Resources, Writing, and Review and Editing. M.S.d.P.: Conceptualization, Methodology, Writing, and Review and Editing, Supervision, Funding Acquisition.
Corresponding author
Ethics declarations
Competing interests
E.E.P., M.C.L., and M.S.d.P. reported receiving grants from Nutrinia Ltd during the conduct of the study. M.S.d.P. reported receiving lecture fees and travel reimbursements from Nutrinia Ltd during the conduct of the study. No other disclosures were reported.
Consent to participate
The signing of the informed consent by the parents or legal guardians was a fundamental criterion for the inclusion of the patient in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moreno-Sanz, B., Lázaro-Perona, F., Escribano, E. et al. Assessment trial of the effect of enteral insulin on the preterm infant intestinal microbiota. Pediatr Res 95, 1117–1123 (2024). https://doi.org/10.1038/s41390-023-02917-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02917-3