Abstract
Background
Gonadotropin-releasing hormone analog (GnRHa) is the standard treatment for children with central precocious puberty (CPP). We assessed efficacy and safety of GnRHa treatment in girls with CPP and early fast puberty (EFP).
Methods
This retrospective observational study included anthropometric, clinical and laboratory data retrieved from medical files of girls with CPP or EFP, treated with GnRHa and followed at a tertiary endocrine clinic during 2007–2021.
Results
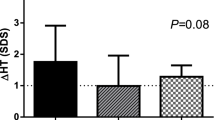
For both CPP (n = 144) and EFP (n = 231) groups, mean height-SDS at GnRHa initiation and termination and at the last follow-up visit was greater than mid-parental height-SDS (P < 0.001). Only among girls with EFP, mean BMI-SDS was higher at treatment termination than initiation (P = 0.025). Median ages at menarche of the CPP and EFP groups were 11.8 and 12.0 years. Menstrual irregularities were reported in 20.3% of girls with CPP and in 18.7% of those with EFP. Adverse effects to treatment were reported in 3.5% and 3.9% of girls with CPP and EFP, respectively.
Conclusions
In this large cohort, GnRHa treatment in girls with EFP was effective without significant adverse effects as in those with CPP. A randomized controlled trial is required to examine the psychological impact of GnRHa treatment of variant early puberty.
Impact statement
-
Gonadotropin-releasing hormone analog (GnRHa) is the standard treatment for central precocious puberty (CPP).
-
We assessed efficacy and safety of GnRHa treatment in girls with early fast puberty (EFP), characterized by pubertal signs between ages 8–9 years with fast pubertal signs advancement and accelerated growth and bone maturation and in girls with CPP.
-
We found in this large cohort that GnRHa treatment in girls with EFP was effective and safe as in those with CPP. A prospective randomized controlled trial is required to examine the psychological impact of GnRHa treatment of variant early puberty.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fuqua, J. S. Treatment and outcomes of precocious puberty: an update. J. Clin. Endocrinol. Metab. 98, 2198–2207 (2013).
Lazar, L., Kauli, R., Pertzelan, A. & Phillip, M. Gonadotropin-suppressive therapy in girls with early and fast puberty affects the pace of puberty but not total pubertal growth or final height. J. Clin. Endocrinol. Metab. 87, 2090–2094 (2002).
Kauli, R. et al. Final height of girls with central precocious puberty, untreated versus treated with cyproterone acetate or GnRH analogue. A comparative study with re-evaluation of predictions by the Bayley-Pinneau method. Horm. Res. 47, 54–61 (1997).
Blumenthal, H. et al. Elevated social anxiety among early maturing girls. Dev. Psychol. 47, 1133–1140 (2011).
Brito, V. et al. Central precocious puberty: revisiting the diagnosis and therapeutic management. Arch. Endocrinol. Metab. 60, 163–172 (2016).
Krishna, K. B. et al. Use of Gonadotropin releasing hormone alalogs in children: update by an international consortium. Horm. Res. Paediatr. 91, 357–372 (2019).
Klein, K. O., Barnes, K. M., Jones, J. V., Feuillan, P. P. & Cutler, G. B. Increased final height in precocious puberty after long-term treatment with LHRH agonists: the National Institutes of Health experience. J. Clin. Endocrinol. Metab. 86, 4711–4716 (2001).
Swaiss, H. H., Khawaja, N. M., Farahid, O. H., Batieha, A. M. & Ajlouni, K. M. Effect of gonadotropin-releasing hormone analogue on final adult height among Jordanian children with precocious puberty. Saudi Med. J. 38, 1101–1107 (2017).
Głąb, E., Wikiera, B., Bieniasz, J. & Barg, E. The influence of GnRH analog therapy on growth in central precocious puberty. Adv. Clin. Exp. Med. 25, 27–32 (2016).
Brito, V. N. et al. Factors determining normal adult height in girls with gonadotropin-dependent precocious puberty treated with depot gonadotropin-releasing hormone analogs. J. Clin. Endocrinol. Metab. 93, 2662–2669 (2008).
Lazar, L., Padoa, A. & Phillip, M. Growth pattern and final height after cessation of gonadotropin-suppressive therapy in girls with central sexual precocity. J. Clin. Endocrinol. Metab. 92, 3483–3489 (2007).
Miller, B. S. & Shukla, A. R. Sterile abscess formation in response to two separate branded long-acting gonadotropin-releasing hormone agonists. Clin. Ther. 32, 1749–1751 (2010).
Guaraldi, F., Beccuti, G., Gori, D. & Ghizzoni, L. Management of endocrine disease: long-term outcomes of the treatment of central precocious puberty. Eur. J. Endocrinol. 174, R79–R87 (2016).
Park, J. & Kim, J. H. Change in body mass index and insulin resistance after 1-year treatment with gonadotropin-releasing hormone agonists in girls with central precocious puberty. Ann. Pediatr. Endocrinol. Metab. 22, 27–35 (2017).
Leite, A. L. et al. Do GnRH agonists really increase body weight gain? Evaluation of a multicentric Portuguese cohort of patients with central precocious puberty. Front. Pediatr. 10, 816635 (2022).
Xiaoping, L. et al. An open label, multicenter clinical trial that investigated the efficacy and safety of leuprorelin treatment of central precocious puberty in Chinese children. Medicine 100, e28158 (2021).
Kim, H. R., Nam, H. K., Rhie, Y. J. & Lee, K. H. Treatment outcomes of gonadotropin-releasing hormone agonist in obese girls with central precocious puberty. Ann. Pediatr. Endocrinol. Metab. 22, 259–265 (2017).
De Sanctis, V., Soliman, A. T., Di Maio, S., Soliman, N. & Elsedfy, H. Long-term effects and significant Adverse Drug Reactions (ADRs) associated with the use of Gonadotropin-Releasing Hormone analogs (GnRHa) for central precocious puberty: a brief review of literature. Acta Biomed. 90, 345–359 (2019).
Willemsen, R. H., Elleri, D., Williams, R. M., Ong, K. K. & Dunger, D. B. Pros and cons of GnRHa treatment for early puberty in girls. Nat. Rev. Endocrinol. 10, 352–363 (2014).
Kuczmarski, R. J. et al. CDC growth charts for the United States: methods and development. Vital-. Health Stat. 246, 1–190 (2002). 11.
Lee, P. A., Luce, M. & Bacher, P. Monitoring treatment of central precocious puberty using basal luteinizing hormone levels and practical considerations for dosing with a 3-month leuprolide acetate formulation. J. Pediatr. Endocrinol. Metab. 29, 1249–1257 (2016).
Bayley, N. & Pinneau, S. Tables for predicting adult height from skeletal age. J. Pediatr. 14, 432–441 (1952).
Borges, M. F. et al. Evaluation of central precocious puberty treatment with GnRH analogue at the Triangulo Mineiro Federal University (UFTM). Arch. Endocrinol. Metab. 59, 515–522 (2015).
López-Miralles, M., Lacomba-Trejo, L., Valero-Moreno, S., Benavides, G. & Pérez-Marín, M. Psychological aspects of pre-adolescents or adolescents with precocious puberty: a systematic review. J. Pediatr. Nurs. 64, e61–e68 (2022).
Michaud, P. A., Suris, J. C. & Deppen, A. Gender-related psychological and behavioral correlates of pubertal timing in a national sample of Swiss adolescents. Mol. Cell Endocrinol. 25, 172–178 (2006).
Tremblay, L. & Frigon, J. Y. Precocious puberty in adolescent girls: a biomarker of later psychosocial adjustment problems. Child Psychiatry Hum. Dev. 36, 73–94 (2005).
Xie, L. L. et al. A clinical study of girls with idiopathic central precocious puberty and psychological behavior problems. Clin. Pediatr. 62, 914–918 (2023).
Shoelwer, M. et al. Psychological assessment of mothers and their daughters at the time of diagnosis of precocious puberty. Int. J. Pediatr. Endocrinol. 5, 1–5 (2015).
Loochi, S. A. et al. Gonadotropin releasing hormone analogue therapy in girls with idiopathic precocious puberty/early-fast puberty: dynamics in adiposity indices, eating habits and quality of life. J. Pediatr. Endocrinol. Metab. 34, 373–383 (2021).
Kendirci, H. N. et al. Evaluating the efficacy of treatment with a GnRH analogue in patients with central precocious puberty. Int. J. Endocrinol. 2015, 247386 (2015).
Knific, T. et al. Final adult height in children with central precocious puberty- a retrospective study. Front. Endocrinol. (Lausanne) 2, 1008474 (2022).
Yoon, J. W., Park, H. A., Lee, J. & Kim, J. H. The influence of gonadotropin-releasing hormone agonists on anthropometric change in girls with central precocious puberty. Korean J. Pediatr. 60, 395–402 (2017).
Arrigo, T. et al. Reduction of baseline body mass index under gonadotropin-suppressive therapy in girls with idiopathic precocious puberty. Eur. J. Endocrinol. 150, 533–537 (2004).
van der Sluis, I. M., Boot, A. M., Krenning, E. P., Drop, S. L. & de Muinck Keizer-Schrama, S. M. Longitudinal follow-up of bone density and body composition in children with precocious or early puberty before, during and after cessation of GnRH agonist therapy. J. Clin. Endocrinol. Metab. 87, 506–512 (2002).
Paterson, W. F., McNeill, E., Young, D. & Donaldson, M. D. Auxological outcome and time to menarche following long-acting goserelin therapy in girls with central precocious or early puberty. Clin. Endocrinol. (Oxf.) 61, 626–634 (2004).
Vuralli, D., Ozon, Z. A., Gonc, E. N., Alikasifoglu, A. & Kandemir, N. Long-term effects of GnRH agonist treatment on body mass index in girls with idiopathic central precocious puberty. J. Pediatr. Endocrinol. Metab. 33, 99–105 (2020).
Wolters, B., Lass, N. & Reinehr, T. Treatment with gonadotropin-releasing hormone analogues: different impact on body weight in normal-weight and overweight children. Horm. Res. Paediatr. 78, 304–311 (2012).
Baek, J. W. et al. Age of menarche and near adult height after long-term gonadotropin-releasing hormone agonist treatment in girls with central precocious puberty. Ann. Pediatr. Endocrinol. Metab. 19, 27–31 (2014).
Lazar, L., Meyerovitch, J., de Vries, L., Phillip, M. & Lebenthal, Y. Treated and untreated women with idiopathic precocious puberty: long-term follow-up and reproductive outcome between the third and fifth decades. Clin. Endocrinol. 80, 570–576 (2014).
Faienza, M. F. et al. Metabolic outcomes, bone health, and risk of polycystic ovary syndrome in girls with idiopathic central precocious puberty treated with gonadotropin-releasing hormone analogues. Horm. Res. Paediatr. 87, 162–169 (2017).
Luo, X. et al. Long-term efficacy and safety of gonadotropin-releasing hormone analog treatment in children with idiopathic central precocious puberty: a systematic review and meta-analysis. Clin. Endocrinol. (Oxf.) 94, 786–796 (2021).
Barros, B. S., Kuschnir, M. C. M. C., Bloch, K. V. & Silva, T. L. N. D. ERICA: age at menarche and its association with nutritional status. J. Pediatr. (Rio J.) 95, 106–111 (2019).
Flash-Luzzatti, S., Weil, C., Shalev, V., Oron, T. & Chodick, G. Long-term secular trends in the age at menarche in Israel: a systematic literature review and pooled analysis. Horm. Res. Paediatr. 81, 266–271 (2014).
Cantas-Orsdemir, S. & Eugster, E. A. Update on central precocious puberty: from etiologies to outcomes. Expert Rev. Endocrinol. Metab. 14, 123–130 (2019).
Tonini, G. & Lazzerini, M. Side effects of GnRH analogue treatment in childhood. J. Pediatr. Endocrinol. Metab. 13, 795–803 (2000).
Gu, Q., Luo, Y., Ye, J. & Shen, X. Comparative efficacy and safety of three current clinical treatments for girls with central precocious puberty: a network meta-analysis. Endocr. Pr. 25, 717–728 (2019).
Kirkgoz, T. et al. Management of systemic hypersensitivity reactions to gonadotropin-releasing hormone analogues during treatment of central precocious puberty. Horm. Res. Paediatr. 93, 66–72 (2020).
Warnock, J. K. & Bundren, J. C. Anxiety and mood disorders associated with gonadotropin-releasing hormone agonist therapy. Psychopharmacol. Bull. 33, 311–316 (1997).
Menk, T. A. S. et al. Assessment of stress levels in girls with central precocious puberty before and during long-acting gonadotropin-releasing hormone agonist treatment: a pilot study. J. Pediatr. Endocrinol. Metab. 30, 657–662 (2017).
Franceschi, R. et al. Prevalence of polycystic ovary syndrome in young women who had idiopathic central precocious puberty. Fertil. Steril. 93, 1185–1191 (2010). 1.
Magiakou, M. A. et al. The efficacy and safety of gonadotropin-releasing hormone analog treatment in childhood and adolescence: a single center, long-term follow-up study. J. Clin. Endocrinol. Metab. 95, 109–117 (2010).
Funding
Research reported in this publication was supported by a research grant from Debiopharm Biopharmaceutical Company. The content is solely the responsibility of the authors.
Author information
Authors and Affiliations
Contributions
L.L.—Substantial contributions to acquisition of data, analysis and interpretation of data, drafting the article, and final approval of the version to be published. M.Y.G.—Substantial contributions to analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published. M.P.—Substantial contributions to acquisition of data, revising the article critically for important intellectual content, and final approval of the version to be published. S.S.—Substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, revising it critically for important intellectual content, and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Subject consent was waived due to the retrospective design.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lerman, L., Yackobovitch-Gavan, M., Phillip, M. et al. Gonadotropin-releasing hormone analogs treatment in girls with central precocious puberty and early fast puberty. Pediatr Res 95, 1051–1059 (2024). https://doi.org/10.1038/s41390-023-02879-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02879-6