Abstract
Background
The Sudden Infant Death Syndrome (SIDS) has been associated with increased peripheral serotonin and an abnormal colonic microbiome, suggesting the colonic metabolome may also be abnormal. This study addresses this potential correlation by comparing colonic autopsy tissue from SIDS to age-matched non-SIDS controls.
Methods
Untargeted metabolomic analysis by mass spectrometry is used to assess human colonic metabolomic differences including serotonin. Expression of genes associated with colonic serotonin synthesis and transport (TPH1, TPH2, DDC, SCL6A4) is measured by qRT-PCR. Microbiome analysis is performed to compare the SIDS and non-SIDS colonic microbiome.
Results
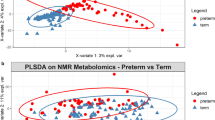
Unsupervised hierarchical cluster and principal component analyses of metabolomic data shows increased variability in the SIDS cohort and separation of SIDS cases from the non-SIDS controls. There is a trend toward increased serotonin in the SIDS cohort but there is no significant difference in expression of the serotonin synthesis and transport genes between SIDS and non-SIDS control cohorts. Microbiome analysis shows no significant difference between the SIDS and non-SIDS control cohorts.
Conclusions
This study demonstrates increased variability in the colonic metabolome and a trend towards increased colonic serotonin in SIDS. The underlying cause of colon metabolomic variability, and its potential role in SIDS pathogenesis, warrants further investigation.
Impact Statement
-
The key message of this article is that SIDS is associated with an aberrant colonic metabolome.
-
This is a novel observation suggesting another component in the pathophysiology underlying SIDS.
-
Investigation of why the colonic metabolome is aberrant may offer new insights to SIDS pathogenesis and new strategies to reduce risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The microbiome analysis FASTQ files are available in the NIH BioProject database under ID PRJNA89917. The MS and qRT-PCR datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Goldstein, R. D. et al. Inconsistent Classification of Unexplained Sudden Deaths in Infants and Children Hinders Surveillance, Prevention and Research: Recommendations from the 3rd International Congress on Sudden Infant and Child Death. Forensic Sci., Med., Pathol. 15, 622–628 (2019).
Kinney, H. C. & Thach, B. T. The Sudden Infant Death Syndrome. N. Engl. J. Med. 361, 795–805 (2009).
Paine, S. M., Jacques, T. S. & Sebire, N. J. Review: Neuropathological Features of Unexplained Sudden Unexpected Death in Infancy: Current Evidence and Controversies. Neuropathol. Appl. Neurobiol. 40, 364–384 (2014).
Kinney, H. C. & Haynes, R. L. The Serotonin Brainstem Hypothesis for the Sudden Infant Death Syndrome. J. Neuropathol. Exp. Neurol. 78, 765–779 (2019).
Haynes, R. L. et al. High Serum Serotonin in Sudden Infant Death Syndrome. Proc. Natl. Acad. Sci. USA 114, 7695–7700 (2017).
Gershon, M. D. & Tack, J. The Serotonin Signaling System: From Basic Understanding to Drug Development for Functional Gi Disorders. Gastroenterology 132, 397–414 (2007).
Chu, D. M. et al. Maturation of the Infant Microbiome Community Structure and Function across Multiple Body Sites and in Relation to Mode of Delivery. Nat. Med. 23, 314–326 (2017).
Leong, L. E. X., Taylor, S. L., Shivasami, A., Goldwater, P. N. & Rogers, G. B. Intestinal Microbiota Composition in Sudden Infant Death Syndrome and Age-Matched Controls. J. Pediatrics 191, 63–68 e61 (2017).
Highet, A. R., Berry, A. M., Bettelheim, K. A. & Goldwater, P. N. Gut Microbiome in Sudden Infant Death Syndrome (Sids) Differs from That in Healthy Comparison Babies and Offers an Explanation for the Risk Factor of Prone Position. Int J. Med. Microbiol 304, 735–741 (2014).
Pearce, J. L., Bettelheim, K. A., Luke, R. K. & Goldwater, P. N. Serotypes of Escherichia Coli in Sudden Infant Death Syndrome. J. Appl. Microbiol. 108, 731–735 (2010).
Highet, A. R. & Goldwater, P. N. Staphylococcal Enterotoxin Genes Are Common in Staphylococcus Aureus Intestinal Flora in Sudden Infant Death Syndrome (Sids) and Live Comparison Infants. FEMS Immunol. Med. Microbiol. 57, 151–155 (2009).
Yano, J. M. et al. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 161, 264–276 (2015).
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the Miseq Illumina Sequencing Platform. Appl. Environ. Microbiol. 79, 5112–5120 (2013).
Schloss, P. D. et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Cameron, E. S., Schmidt, P. J., Tremblay, B. J., Emelko, M. B. & Müller, K. M. Enhancing Diversity Analysis by Repeatedly Rarefying Next Generation Sequencing Data Describing Microbial Communities. Sci. Rep. 11, 22302 (2021).
Zoetendal, E. G. et al. Mucosa-Associated Bacteria in the Human Gastrointestinal Tract Are Uniformly Distributed Along the Colon and Differ from the Community Recovered from Feces. Appl. Environ. Microbiol. 68, 3401–3407 (2002).
Sterling, S. O. & Byard, R. W. Is Academic Rigor in Sudden Infant Death Syndrome (Sids) Research in Decline? Acta Paediatrica 111, 1015–1018 (2022).
Krous, H. F. et al. Sudden Infant Death Syndrome and Unclassified Sudden Infant Deaths: A Definitional and Diagnostic Approach. Pediatrics 114, 234–238 (2004).
Schwarz, E. H. et al. Elevation of Postmortem Triiodothyronine in Sudden Infant Death Syndrome and in Infants Who Died of Other Causes: A Marker of Previous Health. J. Pediatrics 102, 200–205 (1983).
Kaszynski, R. H. et al. Postmortem Interval Estimation: A Novel Approach Utilizing Gas Chromatography/Mass Spectrometry-Based Biochemical Profiling. Anal. Bioanal. Chem. 408, 3103–3112 (2016).
Nearing, J. T. et al. Microbiome Differential Abundance Methods Produce Different Results across 38 Datasets. Nat. Commun. 13, 342 (2022).
Prodan, A. et al. Comparing Bioinformatic Pipelines for Microbial 16s Rrna Amplicon Sequencing. PLoS One 15, e0227434 (2020).
De Livera, A. M. et al. Statistical Methods for Handling Unwanted Variation in Metabolomics Data. Anal. Chem. 87, 3606–3615 (2015).
Schiffman, C. et al. Filtering Procedures for Untargeted Lc-Ms Metabolomics Data. BMC Bioinforma. 20, 334 (2019).
McMurdie, P. J. & Holmes, S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLoS Comput. Biol. 10, e1003531 (2014).
Acknowledgements
Human tissue was obtained from the NIH NeuroBiobank’s Brain and Tissue repository at the University of Maryland, Baltimore. The authors appreciate those who provided consent and assistance with biobanking of these tissues and make this valuable resource available. The authors thank Michelle Dittrick and Kendall Plant for administrative and technical assistance.
Funding
This study is funded by British Columbia Children’s Hospital Research Institute Pathology and Laboratory Medicine SIDS research grants and the authors thank Steps for SIDS and the donors who made these grants possible.
Author information
Authors and Affiliations
Contributions
Both authors (J.T. and R.A.D.) were involved conception and design, acquisition of data, analysis and interpretation of data, drafting and revising the article, and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Terry, J., Dyer, R.A. Aberrant colon metabolome and the sudden infant death syndrome. Pediatr Res 95, 634–640 (2024). https://doi.org/10.1038/s41390-023-02847-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02847-0
This article is cited by
-
Colonic metabolome and SIDS: compared to what?
Pediatric Research (2024)