Abstract
Background
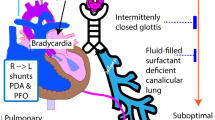
In extremely preterm infants, persistence of cardioventilatory events is associated with long-term morbidity. Therefore, the objective was to characterize physiologic growth curves of apnea, periodic breathing, intermittent hypoxemia, and bradycardia in extremely preterm infants during the first few months of life.
Methods
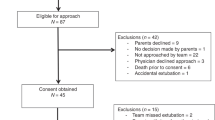
The Prematurity-Related Ventilatory Control study included 717 preterm infants <29 weeks gestation. Waveforms were downloaded from bedside monitors with a novel sharing analytics strategy utilized to run software locally, with summary data sent to the Data Coordinating Center for compilation.
Results
Apnea, periodic breathing, and intermittent hypoxemia events rose from day 3 of life then fell to near-resolution by 8–12 weeks of age. Apnea/intermittent hypoxemia were inversely correlated with gestational age, peaking at 3–4 weeks of age. Periodic breathing was positively correlated with gestational age peaking at 31–33 weeks postmenstrual age. Females had more periodic breathing but less intermittent hypoxemia/bradycardia. White infants had more apnea/periodic breathing/intermittent hypoxemia. Infants never receiving mechanical ventilation followed similar postnatal trajectories but with less apnea and intermittent hypoxemia, and more periodic breathing.
Conclusions
Cardioventilatory events peak during the first month of life but the actual postnatal trajectory is dependent on the type of event, race, sex and use of mechanical ventilation.
Impact
-
Physiologic curves of cardiorespiratory events in extremely preterm-born infants offer (1) objective measures to assess individual patient courses and (2) guides for research into control of ventilation, biomarkers and outcomes.
-
Presented are updated maturational trajectories of apnea, periodic breathing, intermittent hypoxemia, and bradycardia in 717 infants born <29 weeks gestation from the multi-site NHLBI-funded Pre-Vent study.
-
Cardioventilatory events peak during the first month of life but the actual postnatal trajectory is dependent on the type of event, race, sex and use of mechanical ventilation.
-
Different time courses for apnea and periodic breathing suggest different maturational mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are not publicly available due to embargo until after publication of the primary outcome manuscripts, at which point they will become available in the NHLBI’s Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC biolincc.nhlbi.nih.gov). The datasets are available from the corresponding author on reasonable request.
References
Di Fiore, J. M. et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 157, 69–73 (2010).
Di Fiore, J. M. et al. Patterns of oxygenation, mortality, and growth status in the surfactant positive pressure and oxygen trial cohort. J. Pediatr. 186, 49.e41–56.e41 (2017).
Di Fiore, J. M. et al. Early inspired oxygen and intermittent hypoxemic events in extremely premature infants are associated with asthma medication use at 2 years of age. J. Perinatol. 39, 203–211 (2019).
Poets, C. F. et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA 314, 595–603 (2015).
Fairchild, K. D., Nagraj, V. P., Sullivan, B. A., Moorman, J. R. & Lake, D. E. Oxygen desaturations in the early neonatal period predict development of bronchopulmonary dysplasia. Pediatr. Res. 85, 987–993 (2019).
Sullivan, B. A. et al. Early heart rate characteristics predict death and morbidities in preterm infants. J. Pediatr. 174, 57–62 (2016).
Sullivan, B. A. et al. Early pulse oximetry data improves prediction of death and adverse outcomes in a two-center cohort of very low birth weight infants. Am. J. Perinatol. 35, 1331–1338 (2018).
Sullivan, B. A. et al. Clinical and vital sign changes associated with late-onset sepsis in very low birth weight infants at 3 NICUs. J. Neonatal Perinatal Med. 14, 553–561 (2021).
Raffay, T. M. et al. Neonatal intermittent hypoxemia events are associated with diagnosis of bronchopulmonary dysplasia at 36 weeks postmenstrual age. Pediatr. Res. 85, 318–323 (2019).
Vesoulis, Z. A. et al. Early hypoxemia burden is strongly associated with severe intracranial hemorrhage in preterm infants. J. Perinatol. 39, 48–53 (2019).
Fairchild, K. D. et al. Vital signs and their cross-correlation in sepsis and NEC: a study of 1,065 very-low-birth-weight infants in two NICUs. Pediatr. Res. 81, 315–321 (2017).
Nagraj, V. P., Sinkin, R. A., Lake, D. E., Moorman, J. R. & Fairchild, K. D. Recovery from bradycardia and desaturation events at 32 weeks corrected age and NICU length of stay: an indicator of physiologic resilience? Pediatr. Res. 86, 622–627 (2019).
Fairchild, K. et al. Clinical associations of immature breathing in preterm infants: part 1-central apnea. Pediatr. Res. 80, 21–27 (2016).
Patel, M. et al. Clinical associations with immature breathing in preterm infants: part 2-periodic breathing. Pediatr. Res. 80, 28–34 (2016).
Cummings, J. J. & Polin, R. A. Oxygen targeting in extremely low birth weight infants. Pediatrics 138, e1–e9 (2016).
Di Fiore, J. M. et al. Low oxygen saturation target range is associated with increased incidence of intermittent hypoxemia. J. Pediatr. 161, 1047–1052 (2012).
Dennery, P. A. et al. Pre-Vent: the prematurity-related ventilatory control study. Pediatr. Res. 85, 769–776 (2019).
Laird, P. et al. The critical care data exchange format: a proposed flexible data standard for combining clinical and high-frequency physiologic data in critical care. Physiol. Meas. https://doi.org/10.1088/1361-6579/abfc9b (2021).
Vergales, B. D. et al. Accurate automated apnea analysis in preterm infants. Am. J. Perinatol. 31, 157–162 (2014).
Clark, M. T. et al. Stochastic modeling of central apnea events in preterm infants. Physiol. Meas. 37, 463–484 (2016).
Finer, N. N., Higgins, R., Kattwinkel, J. & Martin, R. J. Summary proceedings from the apnea-of-prematurity group. Pediatrics 117, S47–S51 (2006).
Mohr, M. A. et al. Quantification of periodic breathing in premature infants. Physiol. Meas. 36, 1415–1427 (2015).
Di Fiore, J. M. et al. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatr. Res. 72, 606–612 (2012).
Barrington, K. J. & Finer, N. N. Periodic breathing and apnea in preterm infants. Pediatr. Res. 27, 118–121 (1990).
Glotzbach, S. F., Baldwin, R. B., Lederer, N. E., Tansey, P. A. & Ariagno, R. L. Periodic breathing in preterm infants: incidence and characteristics. Pediatrics 84, 785–792 (1989).
Di Fiore, J. M., Martin, R. J. & Gauda, E. B. Apnea of prematurity-perfect storm. Respir. Physiol. Neurobiol. 189, 213–222 (2013).
Gauda, E. B., McLemore, G. L., Tolosa, J., Marston-Nelson, J. & Kwak, D. Maturation of peripheral arterial chemoreceptors in relation to neonatal apnoea. Semin. Neonatol. 9, 181–194 (2004).
Erickson, G., Dobson, N. R. & Hunt, C. E. Immature control of breathing and apnea of prematurity: the known and unknown. J. Perinatol. 41, 2111–2123 (2021).
Poets, C. F. Apnea of prematurity: what can observational studies tell us about pathophysiology? Sleep Med. 11, 701–707 (2010).
Martin, R. J. & Wilson, C. G. Apnea of prematurity. Compr. Physiol. 2, 2923–2931 (2012).
Eichenwald, E. C., Aina, A. & Stark, A. R. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics 100, 354–359 (1997).
Henderson-Smart, D. J. The effect of gestational age on the incidence and duration of recurrent apnoea in newborn babies. Aust. Paediatr. J. 17, 273–276 (1981).
Weintraub, Z. et al. The morphology of periodic breathing in infants and adults. Respir. Physiol. 127, 173–184 (2001).
Fenner, A., Schalk, U., Hoenicke, H., Wendenburg, A. & Roehling, T. Periodic breathing in premature and neonatal babies: incidence, breathing pattern, respiratory gas tensions, response to changes in the composition of ambient air. Pediatr. Res. 7, 174–183 (1973).
Seppä-Moilanen, M., Andersson, S. & Kirjavainen, T. Supplemental oxygen treats periodic breathing without effects on sleep in late-preterm infants. Neonatology 119, 567–574 (2022).
Edwards, B. A., Sands, S. A. & Berger, P. J. Postnatal maturation of breathing stability and loop gain: the role of carotid chemoreceptor development. Respir. Physiol. Neurobiol. 185, 144–155 (2013).
McDonald, F. B., Williams, R., Sheehan, D. & O’Halloran, K. D. Early life exposure to chronic intermittent hypoxia causes upper airway dilator muscle weakness, which persists into young adulthood. Exp. Physiol. 100, 947–966 (2015).
Dylag, A. M. et al. Long-term effects of recurrent intermittent hypoxia and hyperoxia on respiratory system mechanics in neonatal mice. Pediatr. Res. 81, 565–571 (2017).
Di Fiore, J. M., MacFarlane, P. M. & Martin, R. J. Intermittent hypoxemia in preterm infants. Clin. Perinatol. 46, 553–565 (2019).
Vagedes, J., Poets, C. F. & Dietz, K. Averaging time, desaturation level, duration and extent. Arch. Dis. Child. Fetal Neonatal 98, F265–F266 (2013).
Askie, L. M. et al. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA 319, 2190–2201 (2018).
Di Fiore, J. M. et al. Prematurity and postnatal alterations in intermittent hypoxaemia. Arch. Dis. Child. Fetal Neonatal 106, 557–559 (2021).
Schumacher, R. E., Farrell, P. M. & Olson, E. B. Jr Circulating 5-hydroxytryptamine concentrations in preterm newborns. Pediatr. Pulmonol. 3, 117–122 (1987).
Cummings, K. J. & Leiter, J. C. Take a deep breath and wake up: the protean role of serotonin preventing sudden death in infancy. Exp. Neurol. 326, 113165 (2020).
Valeeva, G., Valiullina, F. & Khazipov, R. Excitatory actions of GABA in the intact neonatal rodent hippocampus in vitro. Front. Cell. Neurosci. 7, 20 (2013).
Myers, M. M. et al. Developmental profiles of infant EEG: overlap with transient cortical circuits. Clin. Neurophysiol. 123, 1502–1511 (2012).
Vanhatalo, S. & Kaila, K. Development of neonatal EEG activity: from phenomenology to physiology. Semin. Fetal Neonatal Med. 11, 471–478 (2006).
Carroll, J. L. Developmental plasticity in respiratory control. J. Appl. Physiol. 94, 375–389 (2003).
Bavis, R. W. & MacFarlane, P. M. Developmental plasticity in the neural control of breathing. Exp. Neurol. 287, 176–191 (2017).
Pawar, A., Peng, Y. J., Jacono, F. J. & Prabhakar, N. R. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J. Appl. Physiol. 104, 1287–1294 (2008).
Bavis, R. W., Russell, K. E., Simons, J. C. & Otis, J. P. Hypoxic ventilatory responses in rats after hypercapnic hyperoxia and intermittent hyperoxia. Respir. Physiol. Neurobiol. 155, 193–202 (2007).
Logan, S. et al. Chronic intermittent hyperoxia alters the development of the hypoxic ventilatory response in neonatal rats. Respir. Physiol. Neurobiol. 220, 69–80 (2016).
Esquer, C., Claure, N., D’Ugard, C., Wada, Y. & Bancalari, E. Role of abdominal muscles activity on duration and severity of hypoxemia episodes in mechanically ventilated preterm infants. Neonatology 92, 182–186 (2007).
Kato, I. et al. Developmental characteristics of apnea in infants who succumb to sudden infant death syndrome. Am. J. Respir. Crit. 164, 1464–1469 (2001).
Bairam, A. et al. Sex-based differences in apnoea of prematurity: a retrospective cohort study. Exp. Physiol. 103, 1403–1411 (2018).
Nagraj, V. P., Lake, D. E., Kuhn, L., Moorman, J. R. & Fairchild, K. D. Central apnea of prematurity: does sex matter? Am. J. Perinatol. 38, 1428–1434 (2021).
Dormishian, A., Schott, A., Aguilar, A. C., Bancalari, E. & Claure, N. Pulse oximetry reliability for detection of hypoxemia under motion in extremely premature infants. Pediatr. Res. 93, 118–124 (2023).
Dimaguila, M. A., Di Fiore, J. M., Martin, R. J. & Miller, M. J. Characteristics of hypoxemic episodes in very low birth weight infants on ventilatory support. J. Pediatr. 130, 577–583 (1997).
Chavez, L. & Bancalari, E. Caffeine: some of the evidence behind its use and abuse in the preterm infant. Neonatology 119, 428–432 (2022).
Acknowledgements
The National Institutes of Health (NIH) and the National Heart, Lung, and Blood Institute (NHLBI) provided grant support through cooperative agreements. While NHLBI staff had input into study design, conduct, analysis, and manuscript drafting, the content and views expressed in this article are solely the responsibility of the authors and do not necessarily represent the official views of NIH or the U.S. Department of Health and Human Services. Participating sites collected and stored the data while the University of Virginia (UVa), the lead data and coordinating center (LDCC), analyzed the data. The co-PIs at each site had full access to her/his individual site data and take responsibility for the integrity of the raw waveforms while Dr. Randall Moorman (LDCC co-PI) and Dr. Douglas Lake (LDCC co-PI) take responsibility for the integrity of the data and accuracy of the data analysis. We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following individuals, in addition to those listed as authors, participated in this study: NIH/NHLBI: Neil Aggarwal: NHLBI, Division of Lung Diseases, Bethesda, MD; Lawrence Baizer: NHLBI, Division of Lung Diseases, Bethesda, MD; Peyvand Ghofrani: NHLBI, Division of Lung Diseases, Bethesda, MD; Aaron Laposky: NHLBI, Division of Lung Diseases, Bethesda, MD; Aruna Natarajan: NHLBI, Division of Lung Diseases, Bethesda, MD; Barry Schmetter: NHLBI, Division of Lung Diseases, Bethesda, MD; OSMB: Estelle Gauda (Chair): University of Toronto Hospital for Sick Children, Division of Neonatology, Toronto, Ontario; Jonathan Davis: Tufts Clinical and Translational Science Institute, Division of Newborn Medicine, Boston, MA; Roberta Keller: University of California, San Francisco School of Medicine, Department of Pediatrics, San Francisco CA; Robinder Khemani: Children’s Hospital Los Angeles, Department of Anesthesiology and Critical Care Medicine, Los Angeles, CA; Renee Moore: Drexel University Department of Epidemiology and Biostatistics, Philadelphia, PA; Elliott Weiss: Department of Pediatrics, University of Washington School of Medicine, Seattle, WA; University of Virginia: Amy Camblos: UVa School of Medicine, Clinical Trials Office, UVa School of Medicine, Charlottesville, VA; Gina Duda: UVa School of Medicine, Clinical Trials Office, UVa School of Medicine, Charlottesville, VA; Abigail Flower: UVa, Data Science Institute, Charlottesville, VA; Steven Fowler: UVa School of Medicine, Clinical Trials Office, UVa School of Medicine, Charlottesville, VA; Patcharin Pramoonjago: UVa School of Medicine, Biorepository and Tissue Research Facility, Charlottesville, VA; Craig Rumpel: UVa School of Medicine, Biorepository and Tissue Research Facility, Charlottesville, VA; Northwestern University: Michael Carroll: Ann & Robert H. Lurie Children’s Hospital of Chicago and Stanley Manne Children’s Research Institute, Data Analytics and Reporting, Chicago, IL; Bradley Hopkins: Ann & Robert H. Lurie Children’s Hospital of Chicago, Pediatric Autonomic Medicine, Chicago, IL; University of Alabama at Birmingham: David Paydarfar: University of Texas Austin, Department of Neurology at Dell Medical School, Austin, TX; Elisabeth Salisbury: University of Massachusetts Medical School, Department of Pediatrics and Neurology, Worcester, MA; Bradley Troxler: University of Alabama at Birmingham School of Medicine, Division of Pulmonary and Sleep Medicine, Department of Pediatrics, Birmingham, AL; Washington University: Ryan Colvin: Washington University School of Medicine in St. Louis, Division of General Medicine, St. Louis, MO; Joey Egan: St. Louis Children’s Hospital, Respiratory Care, St. Louis, MO; Elise Eiden: Washington University School of Medicine in St. Louis, Institute for Informatics, St. Louis, MO; Jeffery Hoover: Washington University School of Medicine in St. Louis, Division of Newborn Medicine, St. Louis, MO; Laura Linneman: Washington University School of Medicine in St. Louis, St. Louis, MO; Daniel Mammel: Washington University School of Medicine in St. Louis, Division of Newborn Medicine, St. Louis, MO; Michael McLeland: St. Louis Children’s Hospital, Sleep Laboratory, St. Louis, MO; Harley Pyles: St. Louis Children’s Hospital, Respiratory Care, St. Louis, MO; Barbara Warner: Washington University School of Medicine in St. Louis, Division of Newborn Medicine, St. Louis, MO.
Funding
This work was supported by NIH grants as follows: University of Virginia (NCT03174301): U01 HL133708, HL133708-05S1; Case Western Reserve University: U01 HL133643, The Gerber Foundation; Northwestern University: U01 HL133704; University of Alabama at Birmingham: U01 HL133536; University of Miami: U01 HL133689; Washington University: U01 HL133700.
Author information
Authors and Affiliations
Consortia
Contributions
D.E.W.-M.: substantial contributions to design of study, overseeing enrollment, data collection, safety, analysis and interpretation of data, drafting of work, final approval, agreement to be accountable for all aspects of work including accuracy and integrity. Equal first author. J.D.F.: substantial contributions to design of work and subject recruitment, data collection, safety, analysis and interpretation of data, drafting of work, final approval, agreement to be accountable for all aspects of work including accuracy and integrity. Equal first author. D.L., J.Q., A.M.Z.: substantial contributions to design of work, data analytics, drafting of work, final approval, agreement to be accountable for all aspects of work including accuracy and integrity. A.M.H., N.C., N.A., E.B., J.S.K., J.L.C., R.M., K.K., A.H., P.I., A.D.: substantial contributions to design of study, overseeing enrollment, data collection, and safety, analysis and interpretation of data, drafting of work, final approval, agreement to be accountable for all aspects of work including accuracy and integrity. S.J.R.: substantial contributions to design of study, analysis and interpretation of data, drafting of work, final approval, agreement to be accountable for all aspects of work including accuracy and integrity. N.K.: substantial contributions to design of work, data analytics, interpretation of data, drafting of work, final approval, agreement to be accountable for all aspects of work including accuracy and integrity. P.A.D.: substantial contributions to analysis and interpretation of data, final approval, agreement to be accountable for all aspects of work including accuracy and integrity. J.R.M.: substantial contributions to design of study and work, data analytics, drafting of work, final approval, agreement to be accountable for all aspects of work including accuracy and integrity.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Institutional Review Board (IRB) approval was obtained at all sites. Waiver of consent was approved by the LDCC IRB and 3 of 5 CRC IRBs, while 2 CRC sites obtained consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weese-Mayer, D.E., Di Fiore, J.M., Lake, D.E. et al. Maturation of cardioventilatory physiological trajectories in extremely preterm infants. Pediatr Res 95, 1060–1069 (2024). https://doi.org/10.1038/s41390-023-02839-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02839-0