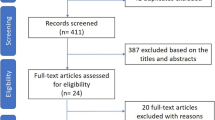
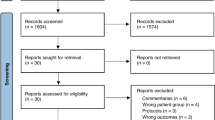
Abstract
Background
This study summarized the available randomized controlled trials (RCTs) to assess the efficacy and safety of macrolides on pathogens, lung function, laboratory parameters, and safety in children with bronchiectasis.
Methods
PubMed, EMBASE, and the Cochrane Library were searched for available papers published up to June 2021. The outcomes were the pathogens, adverse events (AEs), and the forced expiratory volume in one second (FEV1%) predicted.
Results
Seven RCTs (633 participants) were included. The long-term use of macrolides reduced the risk of the presence of Moraxella catarrhalis (RR = 0.67, 95% CI: 0.30–1.50, P = 0.001; I2 = 0.0%, Pheterogeneity = 0.433), but not Haemophilus influenza (RR = 0.19, 95% CI: 0.08–0.49, P = 0.333; I2 = 57.0%, Pheterogeneity = 0.040), Streptococcus pneumonia (RR = 0.91, 95% CI: 0.61–1.35, P = 0.635; I2 = 0.0%, Pheterogeneity = 0.515), Staphylococcus aureus (RR = 1.01, 95% CI: 0.36–2.84, P = 0.986; I2 = 61.9%, Pheterogeneity = 0.033), and any pathogens present (RR = 0.61, 95% CI: 0.29–1.29, P = 0.195; I2 = 80.3%, Pheterogeneity = 0.006). Long-term macrolides had no effect on FEV1% predicted (WMD = 2.61, 95% CI: –1.31, 6.53, P = 0.192; I2 = 0.0%, Pheterogeneity = 0.896). Long-term macrolides did not increase the risk of AEs or serious AEs.
Conclusion
Macrolides do not significantly reduce the risk of pathogens present (except for Moraxella catarrhalis) or increase FEV1% predicted among children with bronchiectasis. Moreover, macrolides were not associated with AEs. Considering the limitations of the meta-analysis, further larger-scale RCTs are needed to confirm the findings.
Impact
-
Macrolides do not significantly reduce the risk of pathogens present (except for Moraxella catarrhalis) among children with bronchiectasis.
-
Macrolides do not significantly increase FEV1% predicted among children with bronchiectasis.
-
This meta-analysis reports on the efficacy and safety of macrolides in the treatment of children with bronchiectasis, providing evidence for the management of children with bronchiectasis.
-
This meta-analysis does not support the use of macrolides in the management of children with bronchiectasis unless the presence of Moraxella catarrhalis is provenor suspected.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Goyal, V., Grimwood, K., Marchant, J., Masters, I. B. & Chang, A. B. Pediatric bronchiectasis: no longer an orphan disease. Pediatr. Pulmonol. 51, 450–469 (2016).
McCallum, G. B. & Binks, M. J. The epidemiology of chronic suppurative lung disease and bronchiectasis in children and adolescents. Front. Pediatr. 5, 27 (2017).
Pasteur, M. C., Bilton, D. & Hill, A. T. British Thoracic Society Non-CF Bronchiectasis Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 65(Suppl 1), i1–i58 (2010).
McShane, P. J., Naureckas, E. T., Tino, G. & Strek, M. E. Non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 188, 647–656 (2013).
El Boustany, P. et al. A review of non-cystic fibrosis bronchiectasis in children with a focus on the role of long-term treatment with macrolides. Pediatr. Pulmonol. 54, 487–496 (2019).
Twiss, J., Metcalfe, R., Edwards, E. & Byrnes, C. New Zealand national incidence of bronchiectasis “too high” for a developed country. Arch. Dis. Child 90, 737–740 (2005).
Das, L. & Kovesi, T. A. Bronchiectasis in children from Qikiqtani (Baffin) Region, Nunavut, Canada. Ann. Am. Thorac. Soc. 12, 96–100 (2015).
Kapur, N., Masters, I. B., Newcombe, P. & Chang, A. B. The burden of disease in pediatric non-cystic fibrosis bronchiectasis. Chest 141, 1018–1024 (2012).
Munro, K. A. et al. Do New Zealand children with non-cystic fibrosis bronchiectasis show disease progression? Pediatr. Pulmonol. 46, 131–138 (2011).
Kapur, N., Masters, I. B. & Chang, A. B. Longitudinal growth and lung function in pediatric non-cystic fibrosis bronchiectasis: what influences lung function stability? Chest 138, 158–164 (2010).
Hill, A. T. et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 74, 1–69 (2019).
Khoo, J. K., Venning, V., Wong, C. & Jayaram, L. Bronchiectasis in the last five years: new developments. J. Clin. Med. 5, 115 (2016).
Haworth, C. S., Bilton, D. & Elborn, J. S. Long-term macrolide maintenance therapy in non-CF bronchiectasis: evidence and questions. Respir. Med. 108, 1397–1408 (2014).
Steel, H. C., Theron, A. J., Cockeran, R., Anderson, R. & Feldman, C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediators Inflamm. 2012, 584262 (2012).
Smith, D. et al. British Thoracic Society guideline for the use of long-term macrolides in adults with respiratory disease. Thorax 75, 370–404 (2020).
Polverino, E. et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 50, 1700629 (2017).
Chang, A. B. et al. European Respiratory Society guidelines for the management of children and adolescents with bronchiectasis. Eur. Respir. J. 58, 2002990 (2021).
Chang, A. B. et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand Thoracic Society of Australia and New Zealand guidelines. Med. J. Aust. 202, 21–23 (2015).
Chalmers, J. D. et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir. Med. 7, 845–854 (2019).
Wang, D., Fu, W. & Dai, J. Meta-analysis of macrolide maintenance therapy for prevention of disease exacerbations in patients with noncystic fibrosis bronchiectasis. Medicine (Baltimore) 98, e15285 (2019).
Kelly, C. et al. Macrolide antibiotics for bronchiectasis. Cochrane Database Syst. Rev. 3, CD012406 (2018).
PRISMA 2020. J. Clin. Epidemiol. 134, A5–A6 (2021).
Swartz, M. K. PRISMA 2020: an Update. J. Pediatr. Health Care 35, 351 (2021).
Aslam, S. & Emmanuel, P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J. Sex. Transm. Dis. AIDS 31, 47–50 (2010).
Sterne, J. A. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898 (2019).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Higgins, J. P. T. et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (Cochrane Collaboration, London, 2020).
Goyal, V. et al. Amoxicillin-clavulanate versus azithromycin for respiratory exacerbations in children with bronchiectasis (BEST-2): a multicentre, double-blind, non-inferiority, randomised controlled trial. Lancet 392, 1197–1206 (2018).
Goyal, V. et al. Efficacy of oral amoxicillin-clavulanate or azithromycin for non-severe respiratory exacerbations in children with bronchiectasis (BEST-1): a multicentre, three-arm, double-blind, randomised placebo-controlled trial. Lancet Respir. Med. 7, 791–801 (2019).
Hare, K. M. et al. Nasopharyngeal carriage and macrolide resistance in Indigenous children with bronchiectasis randomized to long-term azithromycin or placebo. Eur. J. Clin. Microbiol. Infect. Dis. 34, 2275–2285 (2015).
Valery, P. C. et al. Long-term azithromycin for Indigenous children with non-cystic-fibrosis bronchiectasis or chronic suppurative lung disease (Bronchiectasis Intervention Study): a multicentre, double-blind, randomised controlled trial. lancet Respir. Med. 1, 610–620 (2013).
Koh, Y. Y., Lee, M. H., Sun, Y. H., Sung, K. W. & Chae, J. H. Effect of roxithromycin on airway responsiveness in children with bronchiectasis: a double-blind, placebo-controlled study. Eur. Respir. J. 10, 994–999 (1997).
Masekela, R. et al. Lack of efficacy of an immunomodulatory macrolide in childhood HIV related bronchiectasis: a randomised, placebo-controlled trial. J. Antivir. Antiretrovir. 5, 044–049 (2013).
Yalçin, E. et al. Effects of claritromycin on inflammatory parameters and clinical conditions in children with bronchiectasis. J. Clin. Pharm. Ther. 31, 49–55 (2006).
Ramsey, K. A. & Schultz, A. Monitoring disease progression in childhood bronchiectasis. Front. Pediatr. 10, 1010016 (2022).
McDonnell, M. J., Ward, C., Lordan, J. L. & Rutherford, R. M. Non-cystic fibrosis bronchiectasis. QJM 106, 709–715 (2013).
Author information
Authors and Affiliations
Contributions
Conceptualization: G.S. Data curation: M.P., W.L. Formal analysis: M.S., B.Z. Methodology: G.S., S.Y. Writing—original draft: G.S., Y.Z. Writing—review and editing: H.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, G., Zhang, Y., Yu, S. et al. Efficacy and safety of macrolides in the treatment of children with bronchiectasis: a meta-analysis. Pediatr Res 94, 1600–1608 (2023). https://doi.org/10.1038/s41390-023-02591-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02591-5