Abstract
Background
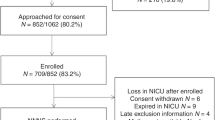
Single-cohort studies have identified distinct neurobehavioral profiles that are associated with prenatal and neonatal factors based on the NICU Network Neurobehavioral Scale (NNNS). We examined socioeconomic, medical, and substance use variables as predictors of NNNS profiles in a multi-cohort study of preterm and term-born infants with different perinatal exposures.
Methods
We studied 1112 infants with a neonatal NNNS exam from the Environmental influences on Child Health Outcomes (ECHO) consortium. We used latent profile analysis to characterize infant neurobehavioral profiles and generalized estimating equations to determine predictors of NNNS profiles.
Results
Six distinct neonatal neurobehavioral profiles were identified, including two dysregulated profiles: a hypo-aroused profile (16%) characterized by lethargy, hypotonicity, and nonoptimal reflexes; and a hyper-aroused profile (6%) characterized by high arousal, excitability, and stress, with low regulation and poor movement quality. Infants in the hypo-aroused profile were more likely to be male, have younger mothers, and have mothers who were depressed prenatally. Infants in the hyper-aroused profile were more likely to be Hispanic/Latino and have mothers who were depressed or used tobacco prenatally.
Conclusions
We identified two dysregulated neurobehavioral profiles with distinct perinatal antecedents. Further understanding of their etiology could inform targeted interventions to promote positive developmental outcomes.
Impact
-
Prior research on predictors of neonatal neurobehavior have included single-cohort studies, which limits generalizability of findings.
-
In a multi-cohort study of preterm and term-born infants, we found six distinct neonatal neurobehavioral profiles, with two profiles being identified as dysregulated.
-
Hypo- and hyper-aroused neurobehavioral profiles had distinct perinatal antecedents.
-
Understanding perinatal factors associated with dysregulated neurobehavior could help promote positive developmental outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets for this manuscript are not publicly available because, per the NIH-approved ECHO Data Sharing Policy, ECHO-wide data have not yet been made available to the public for review/analysis. Requests to access the datasets should be directed to the ECHO Data Analysis Center, ECHO-DAC@rti.org.
References
Liu, J. et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics 125, e90–e98 (2010).
Stephens, B. E. et al. Neurobehavioral assessment predicts motor outcome in preterm infants. J. Pediatr. 156, 366–371 (2010).
Lester, B. M. et al. Infant neurobehavioral dysregulation: behavior problems in children with prenatal substance exposure. Pediatrics 124, 1355–1362 (2009).
Lester, B. M. & Tronick, E. Z. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics 113(Pt. 2), 634–640 (2004).
Hofheimer, J. A. et al. Psychosocial and medical adversity associated with neonatal neurobehavior in infants born before 30 weeks gestation. Pediatr. Res. 87, 721–729 (2020).
McGowan, E. C. et al. Sociodemographic and medical influences on neurobehavioral patterns in preterm infants: a multi-center study. Early Hum. Dev. 142, 104954 (2020).
Zhang, X. et al. The association of prenatal exposure to intensive traffic with early preterm infant neurobehavioral development as reflected by the NICU Network Neurobehavioral Scale (NNNS). Environ. Res. 183, 109204 (2020).
Zhang, X. et al. NICU-based stress response and preterm infant neurobehavior: exploring the critical windows for exposure. Pediatr. Res. https://doi.org/10.1038/s41390-022-01983-3 (2022).
Coyle, M. G. et al. Neurobehavioral effects of treatment for opiate withdrawal. Arch. Dis. Child. Fetal Neonatal Ed. 90, F73–F74 (2005).
Coyle, M. G. et al. Neonatal neurobehavior effects following buprenorphine versus methadone exposure. Addiction 107(Suppl. 1), 63–73 (2012).
Heller, N. A. et al. Neonatal abstinence syndrome: neurobehavior at 6 weeks of age in infants with or without pharmacological treatment for withdrawal. Dev. Psychobiol. 59, 574–582 (2017).
Velez, M. L. et al. Prenatal buprenorphine exposure and neonatal neurobehavioral functioning. Early Hum. Dev. 117, 7–14 (2018).
Velez, M. L., Jansson, L. M., Schroeder, J. & Williams, E. Prenatal methadone exposure and neonatal neurobehavioral functioning. Pediatr. Res. 66, 704–709 (2009).
Camerota, M. et al. Effects of pharmacologic treatment for neonatal abstinence syndrome on DNA methylation and neurobehavior: a prospective cohort study. J. Pediatr. 243, 21–26 (2022).
Lester, B. M. et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics 110, 1182–1192 (2002).
Jones, H. E. et al. Infant neurobehavior following prenatal exposure to methadone or buprenorphine: results from the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Subst. Use Misuse 45, 2244–2257 (2010).
Jones, H. E. et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N. Engl. J. Med. 363, 2320–2331 (2010).
Appleton, A. A. et al. Prenatal programming of infant neurobehaviour in a healthy population. Paediatr. Perinat. Epidemiol. 30, 367–375 (2016).
Sucharew, H. et al. NICU Network Neurobehavioral Scale profiles predict developmental outcomes in a low-risk sample. Paediatr. Perinat. Epidemiol. 26, 344–352 (2012).
Parikh, A. N. et al. Neonatal Intensive Care Unit Network Neurobehavioral Scale profiles in full-term infants: associations with maternal adversity, medical risk, and neonatal outcomes. J. Pediatr. https://doi.org/10.1016/j.jpeds.2022.04.016 (2022).
McGowan, E. C. et al. Analysis of neonatal neurobehavior and developmental outcomes among preterm infants. JAMA Netw. Open 5, e2222249 (2022).
Helderman, J. et al. Association of abnormal findings on neonatal cranial ultrasound with neurobehavior at neonatal intensive care unit discharge in infants born before 30 weeks’ gestation. JAMA Netw. Open 5, e226561 (2022).
Blackwell, C. K., Wakschlag, L. S., Gershon, R. C. & Cella, D. Measurement framework for the Environmental influences on Child Health Outcomes research program. Curr. Opin. Pediatr. 30, 276–284 (2018).
Gillman, M. W. & Blaisdell, C. J. Environmental influences on Child Health Outcomes, a research program of the National Institutes of Health. Curr. Opin. Pediatr. 30, 260–262 (2018).
Jacobson, L. P., Lau, B., Catellier, D. & Parker, C. B. An Environmental influences on Child Health Outcomes viewpoint of data analysis centers for collaborative study designs. Curr. Opin. Pediatr. 30, 269–275 (2018).
Lester, B. M., Tronick, E. Z. & Berry Brazelton, T. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics 113(Suppl. 2), 641–667 (2004).
Lesseur, C., Paquette, A. G. & Marsit, C. J. Epigenetic regulation of infant neurobehavioral outcomes. Med. Epigenet. 2, 71–79 (2014).
Czynski, A. J. et al. Neurodevelopmental outcomes of neonates randomized to morphine or methadone for treatment of neonatal abstinence syndrome. J. Pediatr. 219, 146.e1–151.e1 (2020).
Wouldes, T. A. & Woodward, L. J. Neurobehavior of newborn infants exposed prenatally to methadone and identification of a neurobehavioral profile linked to poorer neurodevelopmental outcomes at age 24 months. PLoS ONE 15, e0240905 (2020).
LeWinn, K. Z. et al. SPR perspectives: Environmental influences on Child Health Outcomes (ECHO) Program: overcoming challenges to generate engaged, multidisciplinary science. Pediatr. Res. https://doi.org/10.1038/s41390-021-01598-0 (2021).
Pineda, R. et al. Randomized clinical trial investigating the effect of consistent, developmentally-appropriate, and evidence-based multisensory exposures in the NICU. J. Perinatol. 41, 2449–2462 (2021).
Ohlsson, A. & Jacobs, S. E. NIDCAP: a systematic review and meta-analyses of randomized controlled trials. Pediatrics 131, e881–e893 (2013).
Marcus, S. et al. Depressive symptoms during pregnancy: Impact on neuroendocrine and neonatal outcomes. Infant Behav. Dev. 34, 26–34 (2011).
Stroud, L. R., McCallum, M. & Salisbury, A. L. Impact of maternal prenatal smoking on fetal to infant neurobehavioral development. Dev. Psychopathol. 30, 1087–1105 (2018).
Duncan, G. J., Lee, K. T. H., Rosales-Rueda, M. & Kalil, A. Maternal age and child development. Demography 55, 2229–2255 (2018).
Fink, N. S., Tronick, E., Olson, K. & Lester, B. Healthy newborns’ neurobehavior: norms and relations to medical and demographic factors. J. Pediatr. 161, 1073.e3–1079.e3 (2012).
Taylor, J. K. Structural racism and maternal health among Black women. J. Law Med. Ethics 48, 506–517 (2020).
Kingsbury, A. M. et al. Social adversity in pregnancy and trajectories of women’s depressive symptoms: a longitudinal study. Women Birth 31, 52–58 (2018).
Kingston, D. et al. Comparison of maternity experiences of canadian-born and recent and non-recent immigrant women: findings from the Canadian Maternity Experiences Survey. J. Obstet. Gynaecol. Can. 33, 1105–1115 (2011).
Law, K. L. et al. Smoking during pregnancy and newborn neurobehavior. Pediatrics 111, 1318–1323 (2003).
Stroud, L. R. et al. Maternal smoking during pregnancy and neonatal behavior: a large-scale community study. Pediatrics 123, e842–e848 (2009).
Song, G. & Pak, V. M. Understanding the effects of prematurity on clinical manifestations of neonatal abstinence syndrome: a narrative literature review. J. Neonatal Nurs. 26, 319–323 (2020).
Ruwanpathirana, R. et al. Prematurity reduces the severity and need for treatment of neonatal abstinence syndrome. Acta Paediatr. 104, e188–e194 (2015).
Wallace, M. E. et al. Racial/ethnic differences in preterm perinatal outcomes. Am. J. Obstet. Gynecol. 216, 306.e1–306.e12 (2017).
Borders, A. E. B. et al. Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. J. Perinatol. 35, 580–584 (2015).
Willer, B. L. & Nafiu, O. O. Racial and ethnic disparities in NICU care practices. Pediatrics 148, e2021051298 (2021).
Wong, H. S. & Edwards, P. Nature or nurture: a systematic review of the effect of socio-economic status on the developmental and cognitive outcomes of children born preterm. Matern. Child Health J. 17, 1689–1700 (2013).
Spittle, A. J. et al. Neurobehaviour and neurological development in the first month after birth for infants born between 32-42 weeks’ gestation. Early Hum. Dev. 96, 7–14 (2016).
Acknowledgements
The authors wish to thank our ECHO colleagues; the medical, nursing, and program staff; and the children and families participating in the ECHO cohorts. We also acknowledge the contribution of the following ECHO program collaborators: ECHO Components—Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL; Data Analysis Center: Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland: Jacobson LP; Research Triangle Institute, Durham, North Carolina: Catellier DJ; Person-Reported Outcomes Core: Northwestern University, Evanston, Illinois: Gershon R, Cella D. ECHO Awardees and Cohorts—Icahn School of Medicine at Mount Sinai, New York, NY: Teitelbaum SL; Emory University, Atlanta, GA: Dunlop A; University of Utah, Salt Lake City, UT: Stanford J.
Funding
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of the Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), UH3OD023320 (J.A.), UH3OD023318 (Dunlop), UH3OD023275 (M.R.K.), UH3OD023347 (B.M.L.), UH3OD023249 (Stanford), UH3OD023348 (T.M.O.). M.C. was additionally supported by a career development award from the National Institute of Mental Health (NIMH), grant K01MH129510 (M.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conception and design of study: M.C., B.M.L. Acquisition of data: J.A., A.S., M.R.K., E.C., S.E.C., P.A.B., B.S.C., J.C., S.A.D., J.B.H., C.R.N., S.L.P., L.M.S. Analysis and interpretation of data: M.C., J.R.K. Drafting the article: M.C., B.M.L. Revising article critically for important intellectual content: M.C., E.C.M., J.A., A.S., M.R.K., E.C., S.E.C., P.A.B., B.S.C., J.C., L.M.D., T.M.E., J.B.H., J.A.H., J.R.K., C.M.L., C.J.M., C.R.N., M.O.S., S.L.P., S.J.S., L.M.S., X.Z., B.M.L. Final approval of the version as submitted: M.C., E.C.M., J.A., A.S., M.R.K., E.C., S.E.C., P.A.B., B.S.C., J.C., L.M.D., S.A.D., T.M.E., J.B.H., J.A.H., J.R.K., C.M.L., C.J.M., C.R.N., M.O.S., S.L.P., S.J.S., L.M.S., X.Z., B.M.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by local Institutional Review Boards, and participants gave informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Camerota, M., McGowan, E.C., Aschner, J. et al. Prenatal and perinatal factors associated with neonatal neurobehavioral profiles in the ECHO Program. Pediatr Res 94, 762–770 (2023). https://doi.org/10.1038/s41390-023-02540-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02540-2
This article is cited by
-
The evolving model of pediatric research
Pediatric Research (2023)