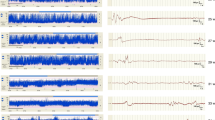
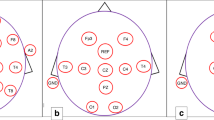
Abstract
The blooming of neonatal neurocritical care over the last decade reflects substantial advances in neuromonitoring and neuroprotection. The most commonly used brain monitoring tools in the neonatal intensive care unit (NICU) are amplitude integrated EEG (aEEG), full multichannel continuous EEG (cEEG), and near-infrared spectroscopy (NIRS). While some published guidelines address individual tools, there is no consensus on consistent, efficient, and beneficial use of these modalities in common NICU scenarios. This work reviews current evidence to assist decision making for best utilization of neuromonitoring modalities in neonates with encephalopathy or with possible seizures. Neuromonitoring approaches in extremely premature and critically ill neonates are discussed separately in the companion paper.
Impact
-
Neuromonitoring techniques hold promise for improving neonatal care.
-
For neonatal encephalopathy, aEEG can assist in screening for eligibility for therapeutic hypothermia, though should not be used to exclude otherwise eligible neonates. Continuous cEEG, aEEG and NIRS through rewarming can assist in prognostication.
-
For neonates with possible seizures, cEEG is the gold standard for detection and diagnosis. If not available, aEEG as a screening tool is superior to clinical assessment alone. The use of seizure detection algorithms can help with timely seizures detection at the bedside.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Austin, T. The development of neonatal neurointensive care. Pediatr. Res. https://doi.org/10.1038/s41390-019-0729-5 (2019).
Glass, H. C., Ferriero, D. M., Rowitch, D. H. & Shimotake, T. K. The neurointensive nursery: concept, development, and insights gained. Curr. Opin. Pediatr. 31, 202–209 (2019).
Bonifacio, S. L. & Van Meurs, K. Neonatal neurocritical care: providing brain-focused care for all at risk neonates. Semin Pediatr. Neurol. 32, 100774 (2019).
Carrasco, M., Stafstrom, C. E., Tekes, A., Parkinson, C. & Northington, F. J. The Johns Hopkins Neurosciences Intensive Care Nursery Tenth Anniversary (2009-2019): a historical reflection and vision for the future. Child Neurol. open 7, 2329048x20907761 (2020).
Dreyfus-Brisac, C. The electroencephalogram of the premature infant and full term newborn:normal and abnormal development of waking and sleeping patterns (Grune and Stratton, 1964).
Clancy, R. R., Bergqvist, A. G. C. & Dlugos, D. J. In Current Practice of Clinical Electroencephalography (Ebersole, J. & Pedley, T. eds.) 160–234 (Lippincott Williams & Wilkins, 2003).
Connell, J. A., Oozeer, R. & Dubowitz, V. Continuous 4-channel EEG monitoring: a guide to interpretation, with normal values, in preterm infants. Neuropediatrics 18, 138–145 (1987).
Holmes, G. L. & Lombroso, C. T. Prognostic value of background patterns in the neonatal EEG. J. Clin. Neurophysiol. 10, 323–352 (1993).
Lamblin, M. D. et al. Electroencephalography of the premature and term newborn. Maturational aspects and glossary. Neurophysiol. Clin. 29, 123–219 (1999).
Mizrahi, E., Hrachovy R & Kellaway, P. Atlas of neonatal electroencephalography 3rd edn (Lippincott Williams & Wilkins, 2004).
Fogtmann, E. P., Plomgaard, A. M., Greisen, G. & Gluud, C. Prognostic accuracy of electroencephalograms in preterm infants: a systematic review. Pediatrics 139, e20161951 (2017).
Shellhaas, R. A. et al. The American clinical neurophysiology society’s guideline on continuous electroencephalography monitoring in neonates. J. Clin. Neurophysiol. 28, 611–617 (2011).
Tsuchida, T. N. et al. American Clinical Neurophysiology Society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society Critical Care Monitoring Committee. J. Clin. Neurophysiol. 30, 161–173 (2013).
El-Dib, M., Chang, T., Tsuchida, T. N. & Clancy, R. R. Amplitude-integrated electroencephalography in neonates. Pediatr. Neurol. 41, 315–326 (2009).
Shah, N. A. & Wusthoff, C. J. How to use: amplitude-integrated EEG (aEEG). Arch. Dis. Child. Educ. Pract. Ed. 100, 75–81 (2015).
Hellstrom-Westas, L., Rosen, I., de Vries, L. S. & Greisen, G. Amplitude-integrated EEG classification and interpretation in preterm and term infants. Neoreviews 7, e76–e87 (2006).
Burdjalov, V. F., Baumgart, S. & Spitzer, A. R. Cerebral function monitoring: a new scoring system for the evaluation of brain maturation in neonates. Pediatrics 112, 855–861 (2003).
Pellicer, A. & Bravo, M. D. C. Near-infrared spectroscopy: a methodology-focused review. Semin. Fetal Neonatal Med. 16, 42–49 (2011).
Naulaers, G. et al. Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. Neonatology 92, 120–126 (2007).
El-Dib, M. & Soul, J. S. Monitoring and management of brain hemodynamics and oxygenation. Handb. Clin. Neurol. 162, 295–314 (2019).
Volpe, J. J. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann. Neurol. 72, 156–166 (2012).
Executive summary: neonatal encephalopathy and neurologic outcome, second edition. report of the American College of Obstetricians and Gynecologists’ task force on neonatal encephalopathy. Obstet. Gynecol. 123, 896–901 (2014).
Chalak, L., Ferriero, D. M., Gressens, P., Molloy, E. & Bearer, C. A 20 years conundrum of neonatal encephalopathy and hypoxic ischemic encephalopathy: are we closer to a consensus guideline? Pediatr. Res. 86, 548–549 (2019).
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Liu, L. et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet 388, 3027–3035 (2016).
Hellstrom-Westas, L., Rosen, I. & Svenningsen, N. W. Predictive value of early continuous amplitude integrated eeg recordings on outcome after severe birth asphyxia in full term infants. Arch. Dis. Child Fetal Neonatal Ed. 72, F34–F38 (1995).
Toet, M. C., Hellstrom-Westas, L., Groenendaal, F., Eken, P. & de Vries, L. S. Amplitude integrated EEG 3 and 6 h after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch. Dis. Child Fetal Neonatal Ed. 81, F19–F23 (1999).
Naqeeb, N., Edwards, A. D., Cowan, F. M. & Azzopardi, D. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics 103, 1263–1271 (1999). al.
ter Horst, H. J. et al. Prognostic significance of amplitude-integrated EEG during the first 72 h after birth in severely asphyxiated neonates. Pediatr. Res. 55, 1026–1033 (2004).
Shalak, L. F., Laptook, A. R., Velaphi, S. C. & Perlman, J. M. Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics 111, 351–357 (2003).
Del Río, R. et al. Amplitude integrated electroencephalogram as a prognostic tool in neonates with hypoxic-ischemic encephalopathy: a systematic review. PLoS One 11, e0165744 (2016).
van Rooij, L. G. et al. Recovery of amplitude integrated electroencephalographic background patterns within 24 h of perinatal asphyxia. Arch. Dis. Child Fetal Neonatal Ed. 90, F245–F251 (2005).
Osredkar, D. et al. Sleep-wake cycling on amplitude-integrated electroencephalography in term newborns with hypoxic-ischemic encephalopathy. Pediatrics 115, 327–332 (2005).
Azzopardi, D. V. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med 361, 1349–1358 (2009).
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365, 663–670 (2005).
Sarkar, S., Barks, J. D. & Donn, S. M. Should amplitude-integrated electroencephalography be used to identify infants suitable for hypothermic neuroprotection? J. Perinatol. 28, 117–122 (2008).
Chandrasekaran, M., Chaban, B., Montaldo, P. & Thayyil, S. Predictive value of amplitude-integrated EEG (aEEG) after rescue hypothermic neuroprotection for hypoxic ischemic encephalopathy: a meta-analysis. J. Perinatol. 37, 684–689 (2017).
Hallberg, B., Grossmann, K., Bartocci, M. & Blennow, M. The prognostic value of early aEEG in asphyxiated infants undergoing systemic hypothermia treatment. Acta Paediatr. 99, 531–536 (2010).
Massaro, A. N. et al. Aeeg evolution during therapeutic hypothermia and prediction of nicu outcome in encephalopathic neonates. Neonatology 102, 197–202 (2012).
Thoresen, M., Hellström-Westas, L., Liu, X. & De Vries, L. S. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics 126, e131–e139 (2010).
Gunn, A. J. & Thoresen, M. Neonatal encephalopathy and hypoxic-ischemic encephalopathy. Handb. Clin. Neurol. 162, 217–237 (2019).
Filan, P. et al. The relationship between the onset of electrographic seizure activity after birth and the time of cerebral injury in utero. BJOG Int. J. Obstet. Gynaecol. 112, 504–507 (2005).
Obeid, R. et al. The correlation between a short-term conventional electroencephalography in the first day of life and brain magnetic resonance imaging in newborns undergoing hypothermia for hypoxic-ischemic encephalopathy. Pediatr. Neurol. 67, 91–97 (2017).
Liu, W. et al. Prognostic value of clinical tests in neonates with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia: a systematic review and meta-analysis. Front Neurol. 11, 133 (2020).
Murray, D. M., Boylan, G. B., Ryan, C. A. & Connolly, S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics 124, e459–e467 (2009).
Nash, K. B. et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology 76, 556–562 (2011).
Wusthoff, C. J. et al. Interrater agreement in the interpretation of neonatal electroencephalography in hypoxic-ischemic encephalopathy. Epilepsia 58, 429–435 (2017).
Massey, S. L. et al. Interrater and intrarater agreement in neonatal electroencephalogram background scoring. J. Clin. Neurophysiol. 36, 1–8 (2019).
Awal, M. A., Lai, M. M., Azemi, G., Boashash, B. & Colditz, P. B. EEG background features that predict outcome in term neonates with hypoxic ischaemic encephalopathy: a structured review. Clin. Neurophysiol. 127, 285–296 (2016).
Korotchikova, I. et al. EEG in the healthy term newborn within 12 h of birth. Clin. Neurophysiol. 120, 1046–1053 (2009).
Kharoshankaya, L. et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev. Med. Child Neurol. 58, 1242–1248 (2016).
Fitzgerald, M. P., Massey, S. L., Fung, F. W., Kessler, S. K. & Abend, N. S. High electroencephalographic seizure exposure is associated with unfavorable outcomes in neonates with hypoxic-ischemic encephalopathy. Seizure 61, 221–226 (2018).
Weeke, L. C. et al. Role of EEG background activity, seizure burden and MRI in predicting neurodevelopmental outcome in full-term infants with hypoxic-ischaemic encephalopathy in the era of therapeutic hypothermia. Eur. J. Paediatr. Neurol. 20, 855–864 (2016).
Glass, H. C. et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology 82, 1239–1244 (2014).
Worden, L. T. et al. The probability of seizures during continuous EEG monitoring in high-risk neonates. Epilepsia 60, 2508–2518 (2019).
Lynch, N. E. et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure 33, 60–65 (2015).
Cornet, M. C. et al. Predictive value of early EEG for seizures in neonates with hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia. Pediatr. Res. 84, 399–402 (2018).
Peng, S. et al. Does near-infrared spectroscopy identify asphyxiated newborns at risk of developing brain injury during hypothermia treatment? Am. J. Perinatol. 32, 555–564 (2015).
Thoresen, M. Cooling the newborn after asphyxia - physiological and experimental background and its clinical use. Semin Neonatol. 5, 61–73 (2000).
Lemmers, P. M. A. et al. Cerebral oxygenation and brain activity after perinatal asphyxia: does hypothermia change their prognostic value? Pediatr. Res. 74, 180–185 (2013).
Rugytė, D. & Strumylaitė, L. Potential relationship between cerebral fractional tissue oxygen extraction (FTOE) and the use of sedative agents during the perioperative period in neonates and infants. Children 7, 209 (2020).
Szakmar, E. et al. Association between cerebral oxygen saturation and brain injury in neonates receiving therapeutic hypothermia for neonatal encephalopathy. J. Perinatol., 41, 269–277 (2021).
Goeral, K. et al. Prediction of outcome in neonates with hypoxic-ischemic encephalopathy ii: role of amplitude-integrated electroencephalography and cerebral oxygen saturation measured by near-infrared spectroscopy. Neonatology 112, 193–202 (2017).
Mitra, S. et al. Relationship between cerebral oxygenation and metabolism during rewarming in newborn infants after therapeutic hypothermia following hypoxic-ischemic brain injury. Adv. Exp. Med Biol. 923, 245–251 (2016).
Archer, L. N., Levene, M. I. & Evans, D. H. Cerebral artery doppler ultrasonography for prediction of outcome after perinatal asphyxia. Lancet 2, 1116–1118 (1986).
Pryds, O., Greisen, G., Lou, H. & Friis-Hansen, B. Vasoparalysis associated with brain damage in asphyxiated term infants. J. Pediatr. 117, 119–125 (1990).
Variane, G. F. T., Chock, V. Y., Netto, A., Pietrobom, R. F. R. & Van Meurs, K. P. Simultaneous near-infrared spectroscopy (Nirs) and amplitude-integrated electroencephalography (aEEG): dual use of brain monitoring techniques improves our understanding of physiology. Front. Pediatrics 7, 560 (2019).
Arriaga-Redondo, M. et al. Lack of variability in cerebral oximetry tendency in infants with severe hypoxic-ischemic encephalopathy under hypothermia. Therapeutic Hypothermia Temp. Manag. 9, 243–250 (2019).
Niezen, C. K., Bos, A. F., Sival, D. A., Meiners, L. C. & Ter Horst, H. J. Amplitude-integrated EEG and cerebral near-infrared spectroscopy in cooled, asphyxiated infants. Am. J. Perinatol. 35, 904–910 (2018).
Pressler, R. M. et al. The ILAE classification of seizures and the epilepsies: modification for seizures in the neonate. position paper by the ilae task force on neonatal seizures. Epilepsia 62, 615–628 (2021).
Wusthoff, C. J. et al. Seizure control in neonates undergoing screening vs confirmatory EEG monitoring. Neurology 97, e587–e596 (2021).
Harris, M. L. et al. Standardized treatment of neonatal status epilepticus improves outcome. J. Child Neurol. 31, 1546–1554 (2016).
Wietstock, S. O., Bonifacio, S. L., McCulloch, C. E., Kuzniewicz, M. W. & Glass, H. C. Neonatal neurocritical care service is associated with decreased administration of seizure medication. J. Child Neurol. 30, 1135–1141 (2015).
Bashir, R. A. et al. Implementation of a neurocritical care program: improved seizure detection and decreased antiseizure medication at discharge in neonates with hypoxic-ischemic encephalopathy. Pediatr. Neurol. 64, 38–43 (2016).
Glass, H. C., Numis, A. L., Gano, D., Bali, V. & Rogers, E. E. Outcomes after acute symptomatic seizures in children admitted to a neonatal neurocritical care service. Pediatr. Neurol. 84, 39–45 (2018).
Pellegrin, S. et al. Neonatal seizures: case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 37, 7596–7609 (2019).
Nunes, M. L. et al. Neonatal seizures: is there a relationship between ictal electroclinical features and etiology? A critical appraisal based on a systematic literature review. Epilepsia open 4, 10–29 (2019).
Cornet, M. C. et al. Neonatal presentation of genetic epilepsies: early differentiation from acute provoked seizures. Epilepsia 62, 1907–1920 (2021).
Shellhaas, R. A., Soaita, A. I. & Clancy, R. R. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics 120, 770–777 (2007).
Zhang, L., Zhou, Y. X., Chang, L. W. & Luo, X. P. Diagnostic value of amplitude-integrated electroencephalogram in neonatal seizures. Neurosci. Bull. 27, 251–257 (2011).
Shellhaas, R. A. & Clancy, R. R. Characterization of neonatal seizures by conventional EEG and single-channel EEG. Clin. Neurophysiol. 118, 2156–2161 (2007).
Shah, D. K. et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous conventional electroencephalography for seizure detection in term infants. Pediatrics 121, 1146–1154 (2008).
Vilan, A. et al. A distinctive ictal amplitude-integrated electroencephalography pattern in newborns with neonatal epilepsy associated with Kcnq2 mutations. Neonatology 112, 387–393 (2017).
Toet, M. C., van der Meij, W., de Vries, L. S., Uiterwaal, C. S. & van Huffelen, K. C. Comparison between simultaneously recorded amplitude integrated electroencephalogram (cerebral function monitor) and standard electroencephalogram in neonates. Pediatrics 109, 772–779 (2002).
Rennie, J. M. et al. Non-expert use of the cerebral function monitor for neonatal seizure detection. Arch. Dis. Child Fetal Neonatal Ed. 89, F37–F40 (2004).
Stevenson, N. J., Lauronen, L. & Vanhatalo, S. The effect of reducing EEG electrode number on the visual interpretation of the human expert for neonatal seizure detection. Clin. Neurophysiol. 129, 265–270 (2018).
Sandoval Karamian, A. G. & Wusthoff, C. J. How helpful is aEEG? Context and user experience matter. Am. J. Perinatol. 39, 1132–1137 (2022).
Van Rooij, L. G. M. et al. Effect of treatment of subclinical neonatal seizures detected with AEEG: randomized, controlled trial. Pediatrics 125, e358–e366 (2010).
Srinivasakumar, P. et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics 136, e1302–e1309 (2015).
Hunt, R. W. et al. Effect of treatment of clinical seizures vs electrographic seizures in full-term and near-term neonates: a randomized clinical trial. JAMA Netw. open 4, e2139604 (2021).
Vanhatalo, S. et al. Why monitor the neonatal brain-that is the important question. Pediatr. Res. https://doi.org/10.1038/s41390-022-02040-9 (2022).
Soul, J. S. et al. Continuous EEG monitoring still recommended for neonatal seizure management: commentary on NEST trial. Pediatr. Res. https://doi.org/10.1038/s41390-022-02138-0 (2022).
Laroia, N., Guillet, R., Burchfiel, J. & McBride, M. C. EEG background as predictor of electrographic seizures in high-risk neonates. Epilepsia 39, 545–551 (1998).
Helmers, S. L. et al. Perioperative electroencephalographic seizures in infants undergoing repair of complex congenital cardiac defects. Electroencephalogr. Clin. Neurophysiol. 102, 27–36 (1997).
Bye, A. M. E. & Flanagan, D. Spatial and temporal characteristics of neonatal seizures. Epilepsia 36, 1009–1016 (1995).
Boylan, G. B. et al. Phenobarbitone, neonatal seizures, and video-EEG. Arch. Dis. Child Fetal Neonatal Ed. 86, F165–F170 (2002).
Scher, M. S., Alvin, J., Gaus, L., Minnigh, B. & Painter, M. J. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr. Neurol. 28, 277–280 (2003).
Wusthoff, C. J. et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Child Neurol. 26, 724–728 (2011).
Sansevere, A. J. et al. Seizure prediction models in the neonatal intensive care unit. J. Clin. Neurophysiol. 36, 186–194 (2019).
Naim, M. Y. et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J. Thorac. Cardiovasc. Surg. 150, 169–180 (2015).
Levy, R. J. et al. Evaluation of seizure risk in infants after cardiopulmonary bypass in the absence of deep hypothermic cardiac arrest. Neurocrit Care 36, 30–38 (2022).
Sands, T. T. et al. Rapid and safe response to low-dose carbamazepine in neonatal epilepsy. Epilepsia 57, 2019–2030 (2016).
Shellhaas, R. A. et al. Profile of neonatal epilepsies: characteristics of a prospective US cohort. Neurology 89, 893–899 (2017).
Painter, M. J. et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N. Engl. J. Med 341, 485–489 (1999).
Sharpe, C. et al. Levetiracetam Versus phenobarbital for neonatal seizures: a randomized controlled trial. Pediatrics 145, e20193182 (2020).
Boylan, G. B., Burgoyne, L., Moore, C., O’Flaherty, B. & Rennie, J. M. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatrica 99, 1150–1155 (2010).
Apers, W. M. J., de Vries, L. S., Groenendaal, F., Toet, M. C. & Weeke, L. C. Delay in treatment of neonatal seizures: a retrospective cohort study. Neonatology 117, 599–605 (2020).
Isaev, D. Y. et al. Attention-based network for weak labels in neonatal seizure detection. Proc. Mach. Learn Res 126, 479–507 (2020).
O’Shea, A., Lightbody, G., Boylan, G. & Temko, A. Neonatal seizure detection from raw multi-channel EEG using a fully convolutional architecture. Neural Netw. 123, 12–25 (2020).
Striano, P. & Minetti, C. Deep learning for neonatal seizure detection: a friend rather than foe. Lancet Child Adolesc. Health 4, 711–712 (2020).
Ansari, A. H. et al. Neonatal seizure detection using deep convolutional neural networks. Int J. Neural Syst. 29, 1850011 (2019).
Stevenson, N., Tapani, K. & Vanhatalo, S. Hybrid neonatal EEG seizure detection algorithms achieve the benchmark of visual interpretation of the human expert. Annu Int Conf. IEEE Eng. Med Biol. Soc. 2019, 5991–5994 (2019).
Pavel, A. M. et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc. Health 4, 740–749 (2020).
Pisani, F. & Spagnoli, C. Outcome in preterm infants with seizures. Handb. Clin. Neurol. 162, 401–414 (2019).
Glass, H. C. et al. Seizures in preterm neonates: a multicenter observational cohort study. J. Pediatr. 72, 19–24 (2017).
Ronen, G. M., Penney, S. & Andrews, W. The epidemiology of clinical neonatal seizures in newfoundland: a population-based study. J. Pediatrics 134, 71–75 (1999).
Lanska, M. J. & Lanska, D. J. Neonatal seizures in the United States: results of the national hospital discharge survey, 1980-1991. Neuroepidemiology 15, 117–125 (1996).
Lanska, M. J., Lanska, D. J., Baumann, R. J. & Kryscio, R. J. A population-based study of neonatal seizures in Fayette County, Kentucky. Neurology 45, 724–732 (1995).
Shah, D. K., Zempel, J., Barton, T., Lukas, K. & Inder, T. E. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr. Res. 67, 102–106 (2010).
Vesoulis, Z. A. et al. Early electrographic seizures, brain injury, and neurodevelopmental risk in the very preterm infant. Pediatr. Res. 75, 564–569 (2013).
Wikström, S. et al. Early single-channel AEEG/EEG predicts outcome in very preterm infants. Acta Paediatrica, Int. J. Paediatrics 101, 719–726 (2012).
Okumura, A. et al. Ictal electroencephalographic findings of neonatal seizures in preterm infants. Brain Dev. 30, 261–268 (2008).
Le Bihannic, A., Beauvais, K., Busnel, A., de Barace, C. & Furby, A. Prognostic value of EEG in very premature newborns. Arch. Dis. Child. Fetal Neonatal Ed. 97, F106–F109 (2012).
Pisani, F. et al. Mortality risk after neonatal seizures in very preterm newborns. J. Child Neurol. 27, 1264–1269 (2012).
Scher, M. S. et al. Electrographic seizures in preterm and full-term neonates: clinical correlates, associated brain lesions, and risk for neurologic sequelae. Pediatrics 91, 128–134 (1993).
Lloyd, R. O., O’Toole, J. M., Pavlidis, E., Filan, P. M. & Boylan, G. B. Electrographic seizures during the early postnatal period in preterm infants. J. Pediatr. 187, 18–25.e12 (2017).
Pisani, F., Barilli, A. L., Sisti, L., Bevilacqua, G. & Seri, S. Preterm infants with video-EEG confirmed seizures: outcome at 30 months of age. Brain Dev. 30, 20–30 (2008).
Janáčková, S. et al. Electroencephalographic characteristics of epileptic seizures in preterm neonates. Clin. Neurophysiol. 127, 2721–2727 (2016).
Weeke, L. C. et al. Rhythmic EEG patterns in extremely preterm infants: classification and association with brain injury and outcome. Clin. Neurophysiol. 128, 2428–2435 (2017).
Pavlidis, E., Lloyd, R. O. & Boylan, G. B. EEG - a valuable biomarker of brain injury in preterm infants. Dev. Neurosci. 39, 23–35 (2017).
Wallois, F., Patil, A., Kongolo, G., Goudjil, S. & Grebe, R. Haemodynamic changes during seizure-like activity in a neonate: a simultaneous AC EEG-SPIR and high-resolution DC EEG recording. Neurophysiol. Clin. 39, 217–227 (2009).
Giorni, C. et al. The usefulness of near-infrared spectroscopy for detecting and monitoring status epilepticus after pediatric cardiac surgery. J. Cardiothorac. Vasc. Anesth. 23, 668–671 (2009).
Arca Diaz, G., Cesaron, E., Alfonso, I., Dunoyer, C. & Yaylali, I. Near infrared spectroscopy in the management of status epilepticus in a young infant. Eur. J. Paediatr. Neurol. 10, 19–21 (2006).
Silas, R., Sehgal, A., Walker, A. M. & Wong, F. Y. Cerebral oxygenation during subclinical seizures in neonatal hypoxic-ischaemic encephalopathy. Eur. J. Paediatr. Neurol. 16, 304–307 (2012).
Sokoloff, M. D., Plegue, M. A., Chervin, R. D., Barks, J. D. & Shellhaas, R. A. Phenobarbital and neonatal seizures affect cerebral oxygen metabolism: a near-infrared spectroscopy study. Pediatr. Res 78, 91–96 (2015).
Funding
M.E.-D. discloses he is the PI of an investigator-initiated research funded by Medtronic and served once on an advisory board for Radiometer. R.M.P. is an investigator for studies with UCB Biosciences, Inc and Johnson & Johnson and has served as a speaker, consultant and/or on advisory boards for Kephala, GW, Natus and UCB Biosciences, Inc. Her research is supported by the National Institute of Health Research (NIHR) Biomedical Research Centre at Great Ormand Street Hospital (GOSH), Cambridge Biomedical Research Centre NIHR and the Evelina Trust. Other authors are disclosing no financial interest.
Author information
Authors and Affiliations
Consortia
Contributions
Substantial contributions to conception and design: All Authors. Drafting the article or revising it critically for important intellectual content: All authors. Final approval of the version to be published: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Dib, M., Abend, N.S., Austin, T. et al. Neuromonitoring in neonatal critical care part I: neonatal encephalopathy and neonates with possible seizures. Pediatr Res 94, 64–73 (2023). https://doi.org/10.1038/s41390-022-02393-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02393-1
This article is cited by
-
Acute symptomatic seizures in newborns: a narrative review
Acta Epileptologica (2024)
-
Quantitative EEG features during the first day correlate to clinical outcome in perinatal asphyxia
Pediatric Research (2024)