Abstract
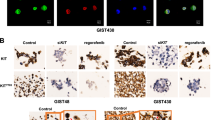
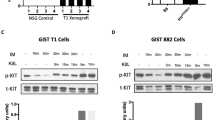
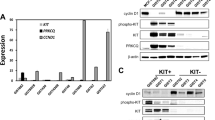
The aberrant activation of RAS/RAF/MEK/ERK signaling is important for KIT mutation-mediated tumorigenesis of gastrointestinal stromal tumor (GIST). In this study, we found that inhibition of RAF1 suppresses the activation of both wild-type KIT and primary KIT mutations in GIST, with primary KIT mutations showing greater sensitivity. This suggests a positive feedback loop between KIT and RAF1, wherein RAF1 facilitates KIT signaling. We further demonstrated that RAF1 associates with KIT and the kinase activity of RAF1 is necessary for its contribution to KIT activation. Accordingly, inhibition of RAF1 suppressed cell survival, proliferation, and cell cycle progression in vitro mediated by both wild-type KIT and primary KIT mutations. Inhibition of RAF1 in vivo suppressed GIST growth in a transgenic mouse model carrying germline KIT/V558A mutation, showing a similar treatment efficiency as imatinib, the first-line targeted therapeutic drug of GIST, while the combination use of imatinib and RAF1 inhibitor further suppressed tumor growth. Acquisition of drug-resistant secondary mutation of KIT is a major cause of treatment failure of GIST following targeted therapy. Like wild-type KIT and primary KIT mutations, inhibition of RAF1 suppressed the activation of secondary KIT mutation, and the cell survival, proliferation, cell cycle progression in vitro, and tumor growth in vivo mediated by secondary KIT mutation. However, the activation of secondary KIT mutation is less dependent on RAF1 compared with that of primary KIT mutations. Taken together, our results revealed that RAF1 facilitates KIT signaling and KIT mutation-mediated tumorigenesis of GIST, providing a rationale for further investigation into the use of RAF1 inhibitors alone or in combination with KIT inhibitor in the treatment of GIST, particularly in cases resistant to KIT inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Data reported in this paper are available from the corresponding author upon reasonable request.
References
Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46.
Nishida T, Yoshinaga S, Takahashi T, Naito Y. Recent Progress and Challenges in the Diagnosis and Treatment of Gastrointestinal Stromal Tumors. Cancers. 2021;13:3158.
Patel SR, Reichardt P. An updated review of the treatment landscape for advanced gastrointestinal stromal tumors. Cancer. 2021;127:2187–95.
Antonescu CR, Sommer G, Sarran L, Tschernyavsky SJ, Riedel E, Woodruff JM, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clinical cancer research : an official journal of the American Association for. Cancer Res. 2003;9:3329–37.
Kang HJ, Nam SW, Kim H, Rhee H, Kim NG, Kim H, et al. Correlation of KIT and platelet-derived growth factor receptor alpha mutations with gene activation and expression profiles in gastrointestinal stromal tumors. Oncogene. 2005;24:1066–74.
Steigen SE, Eide TJ, Wasag B, Lasota J, Miettinen M. Mutations in gastrointestinal stromal tumors-a population-based study from Northern Norway. APMIS. 2007;115:289–98.
Emile JF, Brahimi S, Coindre JM, Bringuier PP, Monges G, Samb P, et al. Frequencies of KIT and PDGFRA mutations in the MolecGIST prospective population-based study differ from those of advanced GISTs. Med Oncol. 2012;29:1765–72.
Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–6.
Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38.
Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302.
Blay JY, Serrano C, Heinrich MC, Zalcberg J, Bauer S, Gelderblom H, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:923–34.
Chen LL, Trent JC, Wu EF, Fuller GN, Ramdas L, Zhang W, et al. A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Res. 2004;64:5913–9.
Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764–74.
Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–90.
Debiec-Rychter M, Cools J, Dumez H, Sciot R, Stul M, Mentens N, et al. Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology. 2005;128:270–9.
Du J, Wang S, Wang R, Wang SY, Han Q, Xu HT, et al. Identifying Secondary Mutations in Chinese Patients with Imatinib-Resistant Gastrointestinal Stromal Tumors (GISTs) by Next Generation Sequencing (NGS). Pathol Oncol Res. 2020;26:91–100.
Cao J, Wei J, Yang P, Zhang T, Chen Z, He F, et al. Genome-scale CRISPR-Cas9 knockout screening in gastrointestinal stromal tumor with Imatinib resistance. Mol Cancer. 2018;17:121.
Huang WK, Akcakaya P, Gangaev A, Lee L, Zeljic K, Hajeri P, et al. miR-125a-5p regulation increases phosphorylation of FAK that contributes to imatinib resistance in gastrointestinal stromal tumors. Exp Cell Res. 2018;371:287–96.
Tsai M, Valent P, Galli SJ. KIT as a master regulator of the mast cell lineage. J allergy Clin Immunol. 2022;149:1845–54.
Lennartsson J, Ronnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiological Rev. 2012;92:1619–49.
Foster BM, Zaidi D, Young TR, Mobley ME, Kerr BA. CD117/c-kit in Cancer Stem Cell-Mediated Progression and Therapeutic Resistance. Biomedicines. 2018;6:31.
Nakahara M, Isozaki K, Hirota S, Miyagawa J, Hase-Sawada N, Taniguchi M, et al. A novel gain-of-function mutation of c-kit gene in gastrointestinal stromal tumors. Gastroenterology. 1998;115:1090–5.
Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Investig. 1993;92:1736–44.
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80.
Bosbach B, Rossi F, Yozgat Y, Loo J, Zhang JQ, Berrozpe G, et al. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc Natl Acad Sci USA. 2017;114:E8448–E57.
Henry JR, Kaufman MD, Peng SB, Ahn YM, Caldwell TM, Vogeti L, et al. Discovery of 1-(3,3-dimethylbutyl)-3-(2-fluoro-4-methyl-5-(7-methyl-2-(methylamino)pyrido[2,3-d]pyrimidin-6-yl)phenyl)urea (LY3009120) as a pan-RAF inhibitor with minimal paradoxical activation and activity against BRAF or RAS mutant tumor cells. J Med Chem. 2015;58:4165–79.
Hall-Jackson CA, Eyers PA, Cohen P, Goedert M, Boyle FT, Hewitt N, et al. Paradoxical activation of Raf by a novel Raf inhibitor. Chem Biol. 1999;6:559–68.
Peng SB, Henry JR, Kaufman MD, Lu WP, Smith BD, Vogeti S, et al. Inhibition of RAF Isoforms and Active Dimers by LY3009120 Leads to Anti-tumor Activities in RAS or BRAF Mutant Cancers. Cancer Cell. 2015;28:384–98.
Zhu G, Shi J, Zhang S, Guo Y, Huang L, Zhao H, et al. Loss of PI3 kinase association improves the sensitivity of secondary mutation of KIT to Imatinib. Cell Biosci. 2020;10:16.
Theou-Anton N, Tabone S, Brouty-Boye D, Saffroy R, Ronnstrand L, Lemoine A, et al. Co expression of SCF and KIT in gastrointestinal stromal tumours (GISTs) suggests an autocrine/paracrine mechanism. Br J Cancer. 2006;94:1180–5.
Blume-Jensen P, Wernstedt C, Heldin CH, Ronnstrand L. Identification of the major phosphorylation sites for protein kinase C in kit/stem cell factor receptor in vitro and in intact cells. J Biol Chem. 1995;270:14192–200.
Blay JY, Kang YK, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Prim. 2021;7:22.
Mol CD, Dougan DR, Schneider TR, Skene RJ, Kraus ML, Scheibe DN, et al. Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J Biol Chem. 2004;279:31655–63.
Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008;3:557–86.
Voytyuk O, Lennartsson J, Mogi A, Caruana G, Courtneidge S, Ashman LK, et al. Src family kinases are involved in the differential signaling from two splice forms of c-Kit. J Biol Chem. 2003;278:9159–66.
Sun J, Pedersen M, Ronnstrand L. The D816V mutation of c-Kit circumvents a requirement for Src family kinases in c-Kit signal transduction. J Biol Chem. 2009;284:11039–47.
Lindblad O, Kazi JU, Ronnstrand L, Sun J. PI3 kinase is indispensable for oncogenic transformation by the V560D mutant of c-Kit in a kinase-independent manner. Cell Mol Life Sci. 2015;72:4399–407.
Sun J, Mohlin S, Lundby A, Kazi JU, Hellman U, Pahlman S, et al. The PI3-kinase isoform p110delta is essential for cell transformation induced by the D816V mutant of c-Kit in a lipid-kinase-independent manner. Oncogene. 2014;33:5360–9.
Campanella NC, Gomes INF, Alves ALV, Leal LF, Evangelista AF, Rosa MN, et al. Biological and therapeutic implications of RKIP in Gastrointestinal Stromal Tumor (GIST): an integrated transcriptomic and proteomic analysis. Cancer Cell Int. 2023;23:256.
Ullah R, Yin Q, Snell AH, Wan L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer Biol. 2022;85:123–54.
Weber CK, Slupsky JR, Kalmes HA, Rapp UR. Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res. 2001;61:3595–8.
Rushworth LK, Hindley AD, O’Neill E, Kolch W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol Cell Biol. 2006;26:2262–72.
Asl ER, Amini M, Najafi S, Mansoori B, Mokhtarzadeh A, Mohammadi A, et al. Interplay between MAPK/ERK signaling pathway and MicroRNAs: A crucial mechanism regulating cancer cell metabolism and tumor progression. Life Sci. 2021;278:119499.
Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Receptor Sig Transduct Res. 2015;35:600–4.
Khan AQ, Kuttikrishnan S, Siveen KS, Prabhu KS, Shanmugakonar M, Al-Naemi HA, et al. RAS-mediated oncogenic signaling pathways in human malignancies. Semin Cancer Biol. 2019;54:1–13.
Holderfield M, Deuker MM, McCormick F, McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer. 2014;14:455–67.
Song Y, Bi Z, Liu Y, Qin F, Wei Y, Wei X. Targeting RAS-RAF-MEK-ERK signaling pathway in human cancer: Current status in clinical trials. Genes Dis. 2023;10:76–88.
Heinrich MC, Rankin C, Blanke CD, Demetri GD, Borden EC, Ryan CW, et al. Correlation of Long-term Results of Imatinib in Advanced Gastrointestinal Stromal Tumors With Next-Generation Sequencing Results: Analysis of Phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017;3:944–52.
Sandoval-Perez A, Winger BA, Jacobson MP. Assessing the Activation of Tyrosine Kinase KIT through Free Energy Calculations. J Chem theory Comput. 2022;18:6251–8.
Blume-Jensen P, Siegbahn A, Stabel S, Heldin CH, Ronnstrand L. Increased Kit/SCF receptor induced mitogenicity but abolished cell motility after inhibition of protein kinase C. EMBO J. 1993;12:4199–209.
Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–23.
MacNicol AM, Muslin AJ, Williams LT. Raf-1 kinase is essential for early Xenopus development and mediates the induction of mesoderm by FGF. Cell. 1993;73:571–83.
Li S, Zhao S, Liang N, Zhang S, Zhang L, Zhou L, et al. SPRY4 inhibits and sensitizes the primary KIT mutants in gastrointestinal stromal tumors (GISTs) to imatinib. Gastric Cancer. 2023;26:677–90.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82373141, 82160521), the Natural Science Foundation of Ningxia province (2023AAC05060, 2023AAC03666), and the General Research Fund of the Research Grants Council of Hong Kong (14112618, 14119120).
Author information
Authors and Affiliations
Contributions
LZ conducted cell experiments, mouse experiments, and analyzed the data; ShZ, XC, JSh, SiZ, JT, KX, MW, CW contributed to molecular and cell biology experiments; JL, LZ and YY contributed to the data analysis and visualization; JSu conceptualized the project, JSu, SL and HZ designed and supervised the study; JSu and LZ drafted the manuscript. JSu, SL, HZ revised the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Zhang, S., Cao, X. et al. RAF1 facilitates KIT signaling and serves as a potential treatment target for gastrointestinal stromal tumor. Oncogene (2024). https://doi.org/10.1038/s41388-024-03063-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-024-03063-8