Abstract
Background
Prenatal fine particulate matter (PM2.5) constituents exposure and reduced fetal growth may be risk factors for accelerated growth in early childhood, an important indicator for lifelong health.
Objective
The study investigated whether the joint effects are present between PM2.5 constituents and reduced fetal growth.
Methods
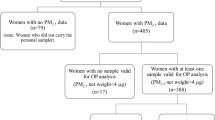
The study was embedded in a birth cohort in China, including 5424 mother-child pairs. Prenatal PM2.5 and its constituents’ [organic carbon (OC), elementary carbon (EC), ammonium (NH4+), nitrate (NO3−), and sulfate (SO42−)] concentrations were estimated based on maternal residential addresses. Fetal growth was evaluated by fetal growth trajectory in utero and preterm birth (PTB), low birth weight (LBW), and small for gestational age (SGA). Children’s accelerated growth was defined as body mass index (BMI) Z-score change of >0.67 between birth and 3 years. Generalized logistic regression was used to analyze the effects of prenatal PM2.5 constituents exposure and fetal growth on children’s accelerated growth. Joint effect was tested on multiplicative scale and additive scale with the relative excess risk due to interaction (RERI).
Results
Children with lower fetal growth trajectory, PTB, LBW, and SGA had increased odds of children’s accelerated growth, with odds ratios (ORs) ranging from 1.704 to 11.605. Compared with lower exposure (≤median), higher exposure (>median) of PM2.5, OC, and SO42− were significantly associated with increased odds of children’s accelerated growth, varying in ORs from 1.163 to 1.478. Prenatal exposure to OC had joint effects with lower fetal growth on children’s accelerated growth. We observed that the interaction was statistically significant on an additive scale in OC and lower fetal growth trajectory (RERI: 0.497, 95% CI: 0.033,0.962).
Impact
Fine particulate matter (PM2.5) is a huge threat to human health worldwide, causing 6.7 million death globally in 2019. According to the theory of DOHaD, prenatal PM2.5 exposure could influence early childhood growth, which is important for lifelong health. We found that prenatal exposure to PM2.5, OC, and SO42− was associated with higher risk of accelerated childhood growth in the first 3 years. More importantly, reduced fetal growth moderated these associations. Our findings highlight the need for policies and interventions on PM2.5 constituents to improve lifelong health, especially for those vulnerable populations with reduced fetal growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Barker DJP. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7.
Matthews EK, Wei J, Cunningham SA. Relationship between prenatal growth, postnatal growth and childhood obesity: a review. Eur J Clin Nutr. 2017;71:919–30.
Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–71.
Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJP. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–53.
Linda SA, Tim JC. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension. 2003;41:451–6.
Mao G, Nachman RM, Sun Q, Zhang X, Koehler K, Chen Z, et al. Individual and joint effects of early-life ambient exposure and maternal prepregnancy obesity on childhood overweight or obesity. Environ Health Perspect. 2017;125:067005.
Chiu Y-HM, Hsu H-HL, Wilson A, Coull BA, Pendo MP, Baccarelli A, et al. Prenatal particulate air pollution exposure and body composition in urban preschool children: examining sensitive windows and sex-specific associations. Environ Res. 2017;158:798–805.
Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, et al. Prenatal exposure to traffic pollution: associations with reduced fetal growth and rapid infant weight gain. Epidemiology. 2015;26:43–50.
Fleisch AF, Luttmann-Gibson H, Perng W, Rifas-Shiman SL, Coull BA, Kloog I, et al. Prenatal and early life exposure to traffic pollution and cardiometabolic health in childhood. Pediatr Obes. 2017;12:48–57.
Sears CG, Mueller-Leonhard C, Wellenius GA, Chen A, Ryan P, Lanphear BP, et al. Early-life exposure to traffic-related air pollution and child anthropometry. Environ Epidemiol. 2019;3:e061.
Patterson WB, Glasson J, Naik N, Jones RB, Berger PK, Plows JF, et al. Prenatal exposure to ambient air pollutants and early infant growth and adiposity in the Southern California Mother’s Milk Study. Environ Health. 2021;20:67.
Starling AP, Moore BF, Thomas DSK, Peel JL, Zhang W, Adgate JL, et al. Prenatal exposure to traffic and ambient air pollution and infant weight and adiposity: the Healthy Start study. Environ Res. 2020;182:109130.
Cho H-J, Lee S-H, Lee S-Y, Kim H-C, Kim H-B, Park MJ, et al. Mid-pregnancy PM2.5 exposure affects sex-specific growth trajectories via ARRDC3 methylation. Environ Res. 2021;200:111640.
Kim JS, Alderete TL, Chen Z, Lurmann F, Rappaport E, Habre R, et al. Longitudinal associations of in utero and early life near-roadway air pollution with trajectories of childhood body mass index. Environ Health. 2018;17:64.
Fossati S, Valvi D, Martinez D, Cirach M, Estarlich M, Fernández-Somoano A, et al. Prenatal air pollution exposure and growth and cardio-metabolic risk in preschoolers. Environ Int. 2020;138:105619.
Rosofsky AS, Fabian MP, Ettinger de Cuba S, Sandel M, Coleman S, Levy JI, et al. Prenatal ambient particulate matter exposure and longitudinal weight growth trajectories in early childhood. Int J Environ Res Public Health. 2020;17:1444.
Fleisch AF, Aris IM, Rifas-Shiman SL, Coull BA, Luttmann-Gibson H, Koutrakis P, et al. Prenatal exposure to traffic pollution and childhood body mass index trajectory. Front Endocrinol. 2019;9:771.
Sun X, Liu C, Liang H, Miao M, Wang Z, Ji H, et al. Prenatal exposure to residential PM2.5 and its chemical constituents and weight in preschool children: a longitudinal study from Shanghai, China. Environ Int. 2021;154:106580.
Moore BF, Starling AP, Martenies SE, Magzamen S, Dabelea D. Joint effects of ambient air pollution and maternal smoking on neonatal adiposity and childhood BMI trajectories in the Healthy Start study. Environ Epidemiol. 2021;5:e142.
Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115:989–95.
Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–50.
Wei Y, Zhang JJ, Li Z, Gow A, Chung KF, Hu M, et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: findings from a natural experiment in Beijing. FASEB J. 2016;30:2115–22.
Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, et al. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012;26:4743–54.
Lin L, Li Q, Yang J, Han N, Jin C, Xu X, et al. The associations of particulate matters with fetal growth in utero and birth weight: a birth cohort study in Beijing, China. Sci Total Environ. 2020;709:136246.
Zhou S, Lin L, Bao Z, Meng T, Wang S, Chen G, et al. The association of prenatal exposure to particulate matter with infant growth: a birth cohort study in Beijing, China. Environ Pollut. 2021;277:116792.
Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol. 1985;151:333–7.
Genolini C, Falissard B. KmL: k-means for longitudinal data. Comput Stat. 2010;25:317–28.
WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand. 1977;56:247–53.
Villar J, Ismail LC, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–68.
World Health Organization. Training course on child growth assessment. Geneva: WHO; 2008.
WHO Multicentre Growth Reference Study Group, de Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;95:76–85.
Duran I, Martakis K, Rehberg M, Stark C, Schafmeyer L, Schönau E. Reference centiles for the evaluation of nutritional status in children using body fat percentage, fat mass and lean body mass index. J Clin Densitom. 2020;23:349–63.
den Dekker HT, Jaddoe VWV, Reiss IK, de Jongste JC, Duijts L. Fetal and infant growth patterns and risk of lower lung function and asthma. The Generation R Study. Am J Respir Crit Care Med. 2018;197:183–92.
Sonnenschein-van der Voort AMM, Jaddoe VWV, Raat H, Moll HA, Hofman A, de Jongste JC, et al. Fetal and infant growth and asthma symptoms in preschool children: the Generation R Study. Am J Respir Crit Care Med. 2012;185:731–7.
Heppe DHM, Kiefte-de Jong JC, Durmuş B, Moll HA, Raat H, Hofman A, et al. Parental, fetal, and infant risk factors for preschool overweight: the Generation R Study. Pediatr Res. 2013;73:120–7.
Chen G, Li S, Knibbs LD, Hamm NAS, Cao W, Li T, et al. A machine learning method to estimate PM(2.5) concentrations across China with remote sensing, meteorological and land use information. Sci Total Environ. 2018;636:52–60.
Hu J, Li X, Huang L, Ying Q, Zhang Q, Zhao B, et al. Ensemble prediction of air quality using the WRF/CMAQ model system for health effect studies in China. Atmos Chem Phys. 2017;17:13103–18.
Skamarock WC, Klemp JB, Dudhia J, Gill DO, Barker DM, Duda MG, et al. A description of the advanced research WRF version 3, NCAR Technical Note. Boulder, CO, USA: National Center for Atmospheric Research; 2008. p. 113.
Guenther A, Jiang X, Heald CL, Sakulyanontvittaya T, Duhl TA, Emmons, et al. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2. 1): an extended and updated framework for modeling biogenic emissions. Geosci Model Dev. 2012;5:1471–92.
Hu J, Wang P, Ying Q, Zhang H, Chen J, Ge X, et al. Modeling biogenic and anthropogenic secondary organic aerosol in China. Atmos Chem Phys. 2017;17:77–92.
Randell H, Gray C, Grace K. Stunted from the start: early life weather conditions and child undernutrition in Ethiopia. Soc Sci Med. 2020;261:113234.
Tutz G, Ramzan S. Improved methods for the imputation of missing data by nearest neighbor methods. Comput Stat Data Anal. 2015;90:84–99.
Tyler JV, Mirjam JK. A tutorial on interaction. Epidemiol Methods. 2014;3:33–72.
Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119:409–15.
Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. 2014.
Yang Y, Ruan Z, Wang X, Yang Y, Mason TG, Lin H, et al. Short-term and long-term exposures to fine particulate matter constituents and health: a systematic review and meta-analysis. Environ Pollut. 2019;247:874–82.
Durmuş B, Mook-Kanamori DO, Holzhauer S, Hofman A, van der Beek EM, Boehm G, et al. Growth in foetal life and infancy is associated with abdominal adiposity at the age of 2 years: the generation R study. Clin Endocrinol. 2010;72:633–40.
Bloomfield FH, Oliver MH, Harding JE. The late effects of fetal growth patterns. Arch Dis Child Fetal Neonatal Ed. 2006;91:F299–304.
Ju L, Hua L, Xu H, Li C, Sun S, Zhang Q, et al. Maternal atmospheric particulate matter exposure and risk of adverse pregnancy outcomes: a meta-analysis of cohort studies. Environ Pollut. 2023;317:120704.
Kroener L, Wang ET, Pisarska MD. Predisposing factors to abnormal first trimester placentation and the impact on fetal outcomes. Semin Reprod Med. 2016;34:027–35.
Turpin BJ, Huntzicker JJ. Identification of secondary organic aerosol episodes and quantitation of primary and secondary organic aerosol concentrations during SCAQS. Atmos Environ. 1995;29:3527–44.
Bové H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, et al. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10:3866.
Breier BH, Vickers MH, Ikenasio BA, Chan KY, Wong WPS. Fetal programming of appetite and obesity. Mol Cell Endocrinol. 2001;185:73–79.
Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506.
Romano M, Guagnano MT, Pacini G, Vigneri S, Falco A, Marinopiccoli M, et al. Association of inflammation markers with impaired insulin sensitivity and coagulative activation in obese healthy women. J Clin Endocrinol Metab. 2003;88:5321–6.
Chang C-J, Jian D-Y, Lin M-W, Zhao J-Z, Ho L-T, Juan C-C. Evidence in obese children: contribution of hyperlipidemia, obesity-inflammation, and insulin sensitivity. PLoS ONE. 2015;10:e0125935.
Chen L, Bell EM, Caton AR, Druschel CM, Lin S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res. 2010;110:162–8.
Pereira G, Bracken MB, Bell ML. Particulate air pollution, fetal growth and gestational length: the influence of residential mobility in pregnancy. Environ Res. 2016;147:269–74.
Funding
The present study was supported by the National Natural Science Foundation of China (Grant No. 81973053). SZ was supported by the China Scholarship Council at the Erasmus University Medical Centre, Rotterdam, The Netherlands (202106010220) as well as the Innovation Fund for Outstanding PhD Candidates of Peking University Health Science Centre.
Author information
Authors and Affiliations
Contributions
SZ: conceptualization, methodology, writing—original draft, formal analysis. TTL, KZ, GBC, YZ, QL, JLH, TL, HR and YMG: methodology, software, writing—review and editing. NH, YLJ, JL and HW: investigation, resources, writing—review and editing. HJW: supervision, conceptualization, project administration, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Institutional Review Board of Peking University Health Science Center (No. IRB00001052-21023).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, S., Li, T., Han, N. et al. The joint effects of prenatal exposure to PM2.5 constituents and reduced fetal growth on children’s accelerated growth in the first 3 years: a birth cohort study. J Expo Sci Environ Epidemiol (2024). https://doi.org/10.1038/s41370-024-00658-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41370-024-00658-x