Abstract
Background
Childhood malnutrition is a major public health issue in Sub-Saharan Africa (SSA) and 61.4 million children under the age of five years in the region are stunted. Although insight from existing studies suggests plausible pathways between ambient air pollution exposure and stunting, there are limited studies on the effect of different ambient air pollutants on stunting among children.
Objective
Explore the effect of early-life environmental exposures on stunting among children under the age of five years.
Methods
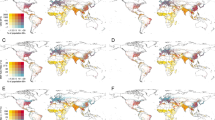
In this study, we used pooled health and population data from 33 countries in SSA between 2006 and 2019 and environmental data from the Atmospheric Composition Analysis Group and NASA’s GIOVANNI platform. We estimated the association between early-life environmental exposures and stunting in three exposure periods – in-utero (during pregnancy), post-utero (after pregnancy to current age) and cumulative (from pregnancy to current age), using Bayesian hierarchical modelling. We also visualise the likelihood of stunting among children based on their region of residence using Bayesian hierarchical modelling.
Results
The findings show that 33.6% of sampled children were stunted. In-utero PM2.5 was associated with a higher likelihood of stunting (OR = 1.038, CrI = 1.002–1.075). Early-life exposures to nitrogen dioxide and sulphate were robustly associated with stunting among children. The findings also show spatial variation in a high and low likelihood of stunting based on a region of residence.
Impact Statement
-
This study explores the effect of early-life environmental exposures on child growth or stunting among sub-Saharan African children. The study focuses on three exposure windows – pregnancy, after birth and cumulative exposure during pregnancy and after birth. The study also employs spatial analysis to assess the spatial burden of stunted growth in relation to environmental exposures and socioeconomic factors. The findings suggest major air pollutants are associated with stunted growth among children in sub-Saharan Africa.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data used in this study are publicly available. The health and demographic data is accessible via the Demographic and Health Survey program data portal (https://dhsprogram.com/Data/). Interested parties have to register on the portal for the data. We do not have permission to share this data. The data for air pollutants and enhanced vegetation index are also accessible through NASA’s GIOVANNI platform (https://giovanni.gsfc.nasa.gov/giovanni/). The PM2.5 dataset is available on the website of the Atmospheric Composition Analysis Group (https://sites.wustl.edu/acag/datasets/surface-pm2-5/).
References
FAO, ECA and AUC. Regional overview of food security and nutrition in Africa: Containing the damage of economic slowdowns and downturns to food security in Africa. Accra, Ghana: FAO, ECA and AUC; 2020. https://doi.org/10.4060/CA7343EN.
UNICEF, WHO & World Bank Group. Levels and trends in child malnutrition: key findings of the 2021 edition of the joint child malnutrition estimates. Geneva; UNICEF, WHO & World Bank Group: 2021.
Stevens GA, Finucane MM, Paciorek CJ, Flaxman SR, White RA, Donner AJ, et al. Trends in mild, moderate, and severe stunting and underweight, and progress towards MDG 1 in 141 developing countries: a systematic analysis of population representative data. Lancet. 2012;380:824–34.
United Nations Department of Economic and Social Affairs. Sustainable development goals. United Nations Department of Economic and Social Affairs; 2015. https://sdgs.un.org/goals
Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–51.
Ewusie JE, Beyene J, Ahiadeke C, Hamid JS. Malnutrition in pre-school children across different geographic areas and socio-demographic groups in Ghana. Matern Child Health J. 2017;21:797–808.
FAO, IFAD, UNICEF, WFP and WHO. The State of Food Security and Nutrition in the World 2019: Safeguarding against economic slowdowns and downturns. Rome: FAO, IFAD, UNICEF, WFP and WHO; 2019.
Sunny BS, DeStavola B, Dube A, Kondowe S, Crampin AC, Glynn JR. Does early linear growth failure influence later school performance? A cohort study in karonga district, Northern Malawi. PLoS One. 2018;13:1–15.
Hoddinott J, Alderman H, Behrman JR, Haddad L, Horton S. The economic rationale for investing in stunting reduction. Matern Child Nutr. 2013;9:69–82.
Adair LS, Fall CHD, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382:525–34.
African Union Commission, NEPAD Planning and Coordinating Agency, UN Economic Commission for Africa, & UN World Food Programme. The cost of hunger in Africa: social and economic impact of child undernutrition in Egypt, Ethiopia, Swaziland and Uganda. Addis Ababa: African Union Commission, NEPAD Planning and Coordinating Agency, UN Economic Commission for Africa, & UN World Food Programme: 2014.
Aheto JMK, Keegan TJ, Taylor BM, Diggle PJ. Childhood malnutrition and its determinants among under‐five children in Ghana. Paediatr Perinat Epidemiol. 2015;29:552–61.
Kismul H, Acharya P, Mapatano MA, Hatløy A. Determinants of childhood stunting in the Democratic Republic of Congo: Further analysis of Demographic and Health Survey 2013-14. BMC Public Health. 2017;18:1–14.
Grace K, Nagle NN, Husak G, Grace K, Nagle NN, Small-scale GHC. Can small-scale agricultural production improve children’s health? Examining stunting vulnerability among very young children in Mali, West Africa. Ann Am Assoc Geogr. 2016;106:722–37.
Brown ME, Grace K, Billing T, Backer D. Considering climate and conflict conditions together to improve interventions that prevent child acute malnutrition. Lancet Planet Heal. 2021;5:e654–e658.
Grace K, Davenport F, Funk C, Lerner AM. Child malnutrition and climate in Sub-Saharan Africa: an analysis of recent trends in Kenya. Appl Geogr. 2012;35:405–13.
Randell H, Gray C, Grace K. Stunted from the start: early life weather conditions and child undernutrition in Ethiopia. Soc Sci Med. 2020;261:113234.
Ahmed KY, Agho KE, Page A, Arora A, Ogbo FA. Mapping geographical differences and examining the determinants of childhood stunting in Ethiopia: a Bayesian geostatistical analysis. Nutrients. 2021;13:2104.
Kinyoki DK, Berkley JA, Moloney GM, Odundo EO, Kandala N, Noor AM. Environmental predictors of stunting among children under-five in Somalia: cross-sectional studies from 2007 to 2010. BMC Public Health. 2016;16:654.
Amegbor PM, Zhang Z, Dalgaard R, Sabel CE. Multilevel and spatial analyses of childhood malnutrition in Uganda: examining individual and contextual factors. Sci Rep. 2020;10:20019.
Grace K, Davenport F, Hanson H, Funk C, Shukla S. Linking climate change and health outcomes: examining the relationship between temperature, precipitation and birth weight in Africa. Glob Environ Chang. 2015;35:125–37.
Davenport F, Grace K, Funk C, Shukla S. Child health outcomes in sub-Saharan Africa: a comparison of changes in climate and socio-economic factors. Glob Environ Chang. 2017;46:72–87.
Alderman H, Headey DD. How important is parental education for child nutrition? World Dev. 2017;94:448–64.
Akombi BJ, Agho KE, Hall JJ, Wali N, Renzaho AMN, Merom D. Stunting, wasting and underweight in Sub-Saharan Africa: a systematic review. Int J Environ Res Public Health. 2017;14:1–18.
Quamme SH, Iversen PO. Prevalence of child stunting in Sub-Saharan Africa and its risk factors. Clin Nutr Open Sci. 2022;42:49–61.
Kinyoki DK, Berkley JA, Moloney GM, Kandala NB, Noor AM. Predictors of the risk of malnutrition among children under the age of 5 years in Somalia. Public Health Nutr. 2015;18:3125–33.
Adedokun ST, Yaya S. Factors associated with adverse nutritional status of children in sub-Saharan Africa: evidence from the demographic and health surveys from 31 countries. Matern Child Nutr. 2021;17:1–10.
Aheto JMK, Dagne GA. Geostatistical analysis, web-based mapping, and environmental determinants of under-5 stunting: evidence from the 2014 Ghana Demographic and Health Survey. Lancet Planet Heal. 2021;5:e347–e355.
Sinharoy SS, Clasen T, Martorell R. Air pollution and stunting: a missing link? Lancet Glob Heal. 2020;8:e472–75.
Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73.
Mandy M, Nyirenda M. Developmental origins of health and disease: the relevance to developing nations. Int Health. 2018;10:66–70.
Barker DJP. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7.
Fisher S, Bellinger DC, Cropper ML, Kumar P, Binagwaho A, Koudenoukpo JB, et al. Air pollution and development in Africa: impacts on health, the economy, and human capital. Lancet Planet Heal. 2021;5:e681–88.
Xie R, Sabel CE, Lu X, Zhu W, Kan H, Nielsen CP, et al. Long-term trend and spatial pattern of PM2.5 induced premature mortality in China. Environ Int. 2016;97:180–6.
WHO. Assessing national capacity for the prevention and control of noncommunicable diseases: report of the 2019 global survey. Geneva: World Health Organization. 2020
Mariet AS, Mauny F, Pujol S, Thiriez G, Sagot P, Riethmuller D, et al. Multiple pregnancies and air pollution in moderately polluted cities: Is there an association between air pollution and fetal growth? Environ Int. 2018;121:890–7.
Goyal N, Karra M, Canning D. Early-life exposure to ambient fine particulate air pollution and infant mortality: pooled evidence from 43 low- and middle-income countries. Int J Epidemiol. 2019;48:1125–41.
Kurata M, Takahashi K, Hibiki A. Gender differences in associations of household and ambient air pollution with child health: evidence from household and satellite-based data in Bangladesh. World Dev. 2020;128:104779.
Pun VC, Dowling R, Mehta S. Ambient and household air pollution on early-life determinants of stunting—a systematic review and meta-analysis. Environ Sci Pollut Res. 2021;28:26404–12.
Spears D, Dey S, Chowdhury S, Scovronick N, Vyas S, Apte J. The association of early-life exposure to ambient PM2.5 and later-childhood height-for-age in India: an observational study. Environ Heal A Glob Access Sci Source. 2019;18:1–10.
Goyal N, Canning D. Exposure to ambient fine particulate air pollution in utero as a risk factor for child stunting in Bangladesh. Int J Environ Res Public Health. 2018;15. https://doi.org/10.3390/ijerph15010022.
Betteridge DJ. What is oxidative stress? Metabolism. 2000;49:3–8.
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017. https://doi.org/10.1155/2017/8416763.
Rider CF, Carlsten C. Air pollution and DNA methylation: effects of exposure in humans. Clin Epigenetics. 2019;11:1–15.
Boamah-Kaali E, Jack DW, Ae-Ngibise KA, Quinn A, Kaali S, Dubowski K, et al. Prenatal and postnatal household air pollution exposure and infant growth trajectories: evidence from a rural Ghanaian pregnancy cohort. Environ Health Perspect. 2021;129:1–12.
Khan JR, Hossain MB, Awan N. Community-level environmental characteristics predictive of childhood stunting in Bangladesh - a study based on the repeated cross-sectional surveys. Int J Environ Health Res. 2022;32:473–86.
Corsi DJ, Neuman M, Finlay JE, Subramanian SV. Demographic and health surveys: a profile. Int J Epidemiol. 2012;41:1602–13.
Amegbor PM, Rosenberg MW. What geography can tell us? Effect of higher education on intimate partner violence against women in Uganda. Appl Geogr. 2019;106:71–81.
UNICEF/WHO/World Bank Group. Levels and trends in child malnutrition: key findings of the 2019 edition of the Joint Child Malnutrition Estimates. UNICEF/WHO/World Bank Group. Geneva: World Health Organization. 2019.
Hammer MS, Van Donkelaar A, Li C, Lyapustin A, Sayer AM, Hsu NC, et al. Global estimates and long-term trends of fine particulate matter concentrations (1998–2018). Environ Sci Technol. 2020;54:7879–90.
Van Donkelaar A, Martin RV, Brauer M, Hsu NC, Kahn RA, Levy RC, et al. Global Annual PM2.5 Grids from MODIS, MISR and SeaWiFS Aerosol Optical Depth (AOD) with GWR, 1998-2016. Palisades NY: NASA Socioeconomic Data and Application Center (SEDAC). 2018. https://doi.org/10.7927/H4ZK5DQS.
World Health Organization. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age - Methods and development. Geneva: WHO; 2006.
Heft-Neal S, Burney J, Bendavid E, Burke M. Robust relationship between air quality and infant mortality in Africa. Nature. 2018;559:254–8.
Amegbor PM. Early-life environmental exposures and anaemia among children under age five in Sub-Saharan Africa: an insight from the demographic & health surveys. Sci Total Environ. 2022;832:154957.
Larsen DA, Grisham T, Slawsky E, Narine L. An individual-level meta-analysis assessing the impact of community-level sanitation access on child stunting, anemia, and diarrhea: evidence from DHS and MICS surveys. PLoS Negl Trop Dis. 2017;11:1–13.
Amadu I, Seidu AA, Duku E, Boadu Frimpong J, Hagan JE Jr, Aboagye RG, et al. Risk factors associated with the coexistence of stunting, underweight, and wasting in children under 5 from 31 sub-Saharan African countries. BMJ Open. 2021;11:1–10.
Chen Q, Elliott MR, Little RJA. Bayesian penalized spline model-based inference for finite population proportion in unequal probability sampling. Surv Methodol. 2010;36:23–34.
Vandendijck Y, Faes C, Kirby RS, Lawson A, Hens N. Model-based inference for small area estimation with sampling weights. Spat Stat. 2016;18:455–73.
Wooldridge JM. Introductory econometrics. 5th ed. Mason, OH: South-Western; 2013.
van Niekerk J, Bakka H, Rue H, Schenk O. New frontiers in Bayesian modeling using the INLA package in R. J Stat Softw. 2021;100. https://doi.org/10.18637/jss.v100.i02.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. https://www.R-project.org/.
Bakka H, Fuglstad G, Riebler A, Bolin D, Krainski E, Simpson D, et al. Spatial modelling with R-INLA: a review. WIREs Comput Stat. 2018; 10:e1443. https://doi.org/10.1002/wics.1443.
Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc Ser B Stat Methodol. 2009;71:319–92.
Martino S, Akerkar R, Rue H. Approximate Bayesian Inference for Survival Models. Scand J Stat. 2011;38:514–28.
Wickham H. ggplot2: elegant graphics for data analysis. New York, NY: Springer-Verlag; 2016.
van den Brand T. ggh4x: hacks for ‘ggplot2’. 2022. R package version 0.2.3.
Tennekes M. tmap: thematic maps in R. J Stat Softw. 2018;84:1–39.
Krainski ET, Gómez-Rubio V, Bakka H, Lenzi A, Castro-Camilo D, Simpson D. et al. Advanced spatial differential equations stochastic partial modeling with using R and INLA. New York: Chapman and Hall/CRC; 2019. https://doi-org.ez.statsbiblioteket.dk:12048/10.1201/9780429031892.
World Health Organization. WHO global air quality guidelines. Particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. Geneva: WHO; 2021.
Butenhoff CL, Khalil MAK, Porter WC, Al-Sahafi MS, Almazroui M, Al-Khalaf A. Evaluation of ozone, nitrogen dioxide, and carbon monoxide at nine sites in Saudi Arabia during 2007. J Air Waste Manag Assoc. 2015;65:871–86.
Strak M, Weinmayr G, Rodopoulou S, Chen J, De Hoogh K, Andersen ZJ et al. Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: pooled analysis. BMJ. 2021; 374. https://doi.org/10.1136/bmj.n1904.
WHO. Health aspects of air pollution with particulate matter, ozone and nitrogen dioxide. Bonn, Germany: WHO; 2003.
Sitaras IE, Siskos PA. The role of primary and secondary air pollutants in atmospheric pollution: Athens urban area as a case study. Environ Chem Lett. 2008;6:59–69.
O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, et al. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003;111:1861–70.
Macintyre S, Ellaway A, Cummins S. Place effects on health: How can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;55:125–39.
Cozier YC. Invited commentary: the enduring role of ‘place’ in health-a historic perspective. Am J Epidemiol. 2017;185:1203–5.
Acknowledgements
Prince M. Amegbor and Clive E. Sabel were supported by Big Data Centre for Environment and Health (BERTHA) funded by the Novo Nordisk Foundation Challenge Programme (grant NNF17OC0027864). Mark W. Rosenberg is the Tier I Canada Research Chair in Aging, Health and Development and supported by funding from the Canada Research Chairs program. We are also grateful to the NASA’s Goddard Earth Sciences Data and Information Services Center (GES DISC), the USAID DHS programme, and Atmospheric Composition Analysis Group at Dalhousie University and Washington University for the data used in this study.
Funding
There is no funding for this research; however, the authors wish to acknowledge support from the Big Data Centre for Environment and Health (BERTHA) funded by the Novo Nordisk Foundation Challenge Programme (grant NNF17OC0027864).
Author information
Authors and Affiliations
Contributions
PMA, CES, AJM, LHM and MWR designed and conceptualised the study. PMA curated data and developed statistical methods. PMA, CES, AJM, LHM and MWR were involve in the analysis and investigation. PMA wrote the original draft with supervision from CES, AJM, LHM and MWR. All authors contributed to reviewing, revising and editing the manuscript. All authors had access to all data used in the study. All authors had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Ethical approval
This is an observational study. The DHS surveys including the procedures and questionnaires were reviewed and approved by the ICF Institutional Review Board (IRB). Additionally, ethical review was conducted by an IRB in the host country for the surveys. ICF IRB ensures that the survey complies with the U.S. Department of Health and Human Services regulations for the protection of human subjects (45 CFR 46), while the host country IRB ensures that the survey complies with laws and norms of the nation. No further ethics approval was needed for this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amegbor, P.M., Sabel, C.E., Mortensen, L.H. et al. Early-life air pollution and green space exposures as determinants of stunting among children under age five in Sub-Saharan Africa. J Expo Sci Environ Epidemiol (2023). https://doi.org/10.1038/s41370-023-00572-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41370-023-00572-8
Keywords
This article is cited by
-
Impacts of ambient air pollution exposure on child growth in East African countries
Air Quality, Atmosphere & Health (2024)