Abstract
Background:
We previously reported the diagnostic efficacy of the age- and prostate volume-adjusted prostate biopsy method (the adjusted biopsy method). Here, we developed a new nomogram for predicting cancer probability at initial biopsy using the adjusted method.
Methods:
Between 2002 and 2010, 1059 Japanese men with PSA levels between 1.1 and 40 ng ml−1 and biopsied for the first time using the adjusted method at Gunma University Hospital were enrolled. All subjects underwent digital rectal examination (DRE) and transrectal ultrasonography (TRUS). Data from the initial 849 subjects were used for development of the nomogram and those from the final 210 subjects were used for internal validation. External validation was conducted using data from two affiliated hospitals where the same adjusted biopsy method was used. The nomogram was developed through logistic regression analysis, and predictive accuracy and performance characteristics were assessed using the area under the curve (AUC) of the receiver operating characteristics and calibration plots. Furthermore, we compared the predictive accuracy of the newly developed nomogram with the ‘Prostate Risk Indicator’ using the development data set, as well as the two external data sets.
Results:
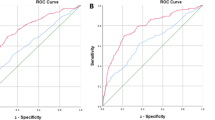
The AUC of the logistic regression-based nomogram was significantly higher than those of any single clinical parameter. External validation showed significant correlations with the present model. The AUC-receiver operating characteristic of the ‘Prostate Risk Indicator’ was the second largest following the new nomogram using the development data set and one external data set and almost equal to the new nomogram using the other external data set.
Conclusions:
Nevertheless the present model does not include somewhat subjective findings on TRUS abnormality, which is necessary for the estimation by ‘Prostate Risk Indicator’, the predictive accuracy of the present simple nomogram could be excellent enough to contribute to accurate shared decision-making between doctors and men who are candidates for the adjusted biopsy scheme.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360: 1320–1328.
Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010; 11: 725–732.
Vashi AR, Wojno KJ, Gillespie B, Oesterling JE . A model for the number of cores per prostate biopsy based on patient age and prostate gland volume. J Urol 1998; 159: 920–924.
Ito K, Ohi M, Yamamoto T, Miyamoto S, Kurokawa K, Fukabori Y et al. The diagnostic accuracy of the age-adjusted and prostate volume-adjusted biopsy method in males with prostate specific antigen levels of 4.1–10.0 ng/ml. Cancer 2002; 95: 2112–2119.
Eastham JA, May R, Robertson JL, Sartor O, Kattan MW . Development of a nomogram that predicts the probability of a positive prostate biopsy in men with an abnormal digital rectal examination and a prostate-specific antigen between 0 and 4 ng/ml. Urology 1999; 54: 709–713.
Karakiewicz PI, Benayoun S, Kattan MW, Perrotte P, Valiquette L, Scardino PT et al. Development and validation of a nomogram predicting the outcome of prostate biopsy based on patient age, digital rectal examination and serum prostate specific antigen. J Urol 2011; 173: 1930–1934.
Suzuki H, Komiya A, Kamiya N, Imamoto T, Kawamura K, Miura J et al. Development of a nomogram to predict probability of positive initial prostate biopsy among Japanese patients. Urology 2006; 67: 131–136.
Nam RK, Toi A, Klotz LH, Trachtenberg J, Jewett MA, Appu S et al. Assessing individual risk for prostate cancer. J Clin Oncol 2007; 25: 3582–3588.
Chun FK, Briganti A, Graefen M, Montorsi F, Porter C, Scattoni V et al. Development and validation of an extended 10-core biopsy nomogram. Eur Urol 2007; 52: 436–445.
Kawakami S, Koga F, Fujii Y, Saito K, Yamamoto S, Tatokoro M et al. History of malignancy is a predictor of prostate cancer detection: incorporation into a pre-biopsy nomogram. Int J Urol 2008; 15: 1055–1060.
Kawakami S, Numao N, Okubo Y, Koga F, Yamamoto S, Saito K et al. Development, validation, and head-to-head comparison of logistic regression-based nomograms and artificial neural network models predicting prostate cancer on initial extended biopsy. Eur Urol 2008; 54: 610–611.
Kawamura K, Suzuki H, Kamiya N, Imamoto T, Yano M, Miura J et al. Development of a new nomogram for predicting the probability of a positive initial prostate biopsy in Japanese patients with serum PSA levels less than 10 ng/ml. Int J Urol 2008; 15: 598–603.
Ide H, Yasuda M, Nishio K, Saito K, Isotani S, Kamiyama Y et al. Development of a nomogram for predicting high-grade prostate cancer on biopsy: the significance of serum testosterone levels. Anticancer Res 2007; 28: 2487–2492.
Scattoni V, Raber M, Abdollah F, Roscigno M, Dehò F, Angiolilli D et al. Biopsy schemes with the fewest cores for detecting 95% of the prostate cancers detected by a 24-core biopsy. Eur Urol 2010; 57: 1–8.
Roobol MJ, Steyerberg EW, Kranse R, Wolters T, van den Bergh RC, Bangma CH et al. A risk-based strategy improves prostate-specific antigen-driven detection of prostate cancer. Eur Urol 2010; 57: 79–85.
Schmid HP, McNeal JE, Stamey TA . Observations on the doubling time of prostate cancer. The use of serial prostate-specific antigen in patients with untreated disease as a measure of increasing cancer volume. Cancer 1993; 71: 2031–2040.
Dugan JA, Bostwick DG, Myers RP, Qian J, Bergstralh EJ, Oesterling JE . The definition and preoperative prediction of clinically insignificant prostate cancer. JAMA 1996; 275: 288–294.
Ito K, Yamamoto T, Kubota Y, Suzuki K, Fukabori Y, Kurokawa K et al. Usefulness of age-specific reference range of prostate-specific antigen for Japanese men older than 60 years in mass screening for prostate cancer. Urology 2000; 56: 278–282.
Eskew LA, Bare RL, McCullough DL . Systematic 5 region biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol 1997; 157: 199–202.
Presti JC, Chang JJ, Bhargava V, Shinohara K . The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol 2000; 163: 163–167.
Gore JL, Shariat SF, Miles BJ, Kadmon D, Jiang N, Wheeler TM et al. Optimal combinations of systematic sextant and laterally directed biopsies for the detection of prostate cancer. J Urol 2001; 165: 1554–1559.
Guichard G, Larre S, Gallina A, Lazar A, Faucon H, Chemama S et al. Extended 21-sample needle biopsy protocol for diagnosis of prostate cancer in 1000 consecutive patients. Eur Urol 2007; 52: 430–435.
Chun FK, Epstein JI, Ficarra V, Freedland SJ, Montironi R, Montorsi F et al. Optimizing performance and interpretation of prostate biopsy: a critical analysis of the literature. Eur Urol 2010; 58: 851–864.
Uzzo RG, Wei JT, Waldbaum RS, Perlmutter AP, Byrne JC, Vaughan Jr ED . The influence of prostate size on cancer detection. Urology 1995; 46: 831–836.
de la Taille A, Antiphon P, Salomon L, Cherfan M, Porcher R, Hoznek A et al. Prospective evaluation of a 21-sample needle biopsy procedure designed to improve the prostate cancer detection rate. Urology 2003; 61: 1181–1186.
Scattoni V, Roscigno M, Raber M, Dehò F, Maga T, Zanoni M et al. Initial extended transrectal prostate biopsy—are more prostate cancers detected with 18 cores than 12 cores? J Urol 2008; 179: 1327–1331.
Remzi M, Fong YK, Dobrovits M, Anagnostou T, Seitz C, Waldert M et al. The Vienna nomogram: validation of a novel biopsy strategy defining the optimal number of cores based on patient age and total prostate volumes. J Urol 2005; 174: 1256–1261.
Jiang J, Colli J, El-Galley R . A simple method for estimating the optimal number of prostate biopsy cores needed to maintain high cancer detection rates while minimizing unnecessary biopsy sampling. J Endourol 2010; 24: 143–147.
Bassler TJ, Orozco R, Bassler GJ, O’Dowd GJ, Stamey TA . Most prostate cancers missed by raising the upper limit of normal prostate-specific antigen for men in their sixties are clinically significant. Urology 1998; 52: 1064–1069.
Ohigashi T, Kanao K, Mizuno R, Kikuchi E, Nakashima J, Oya M . Predicting the probability of significant prostate cancer in Japanese men with serum prostate-specific antigen less than 10 ng/ml: development of a novel pre-biopsy nomogram. Int J Urol 2010; 17: 274–280.
Miyakubo M, Ito K, Yamamoto T, Takechi H, Ohi M, Suzuki K . Prostate-specific antigen: its usefulness in the era of multiple-core prostate biopsy. Int J Urol 2009; 16: 561–565.
Acknowledgements
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nomura, M., Ito, K., Miyakubo, M. et al. Development and external validation of a nomogram for predicting cancer probability at initial prostate biopsy using the life expectancy- and prostate volume-adjusted biopsy scheme. Prostate Cancer Prostatic Dis 15, 202–209 (2012). https://doi.org/10.1038/pcan.2011.62
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2011.62
Keywords
This article is cited by
-
Prostate cancer in Asian men
Nature Reviews Urology (2014)
-
Prostate Cancer Nomograms: A Review of Their Use in Cancer Detection and Treatment
Current Urology Reports (2014)
-
Diagnostic significance of [−2]pro-PSA and prostate dimension-adjusted PSA-related indices in men with total PSA in the 2.0–10.0 ng/mL range
World Journal of Urology (2013)
-
Role of squalene synthase in prostate cancer risk and the biological aggressiveness of human prostate cancer
Prostate Cancer and Prostatic Diseases (2012)