Abstract
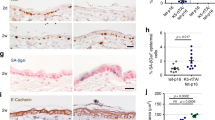
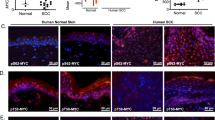
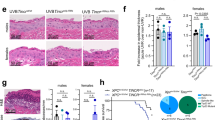
To define a functional role for the endosomal/lysosomal cysteine protease cathepsin L (Ctsl) during squamous carcinogenesis, we generated mice harboring a constitutive Ctsl deficiency in addition to epithelial expression of the human papillomavirus type 16 oncogenes (human cytokeratin 14 (K14)–HPV16). We found enhanced tumor progression and metastasis in the absence of Ctsl. As tumor progression in K14–HPV16 mice is dependent on inflammation and angiogenesis, we examined immune cell infiltration and vascularization without finding any effect of the Ctsl genotype. In contrast, keratinocyte-specific transgenic expression of cathepsin V, the human orthologue of mouse Ctsl, in otherwise Ctsl-deficient K14–HPV16 mice restored the phenotype observed in the control HPV16 skin. To better understand this phenotype at the molecular level, we measured several oncogenic signal transduction pathways in primary keratinocytes on stimulation with keratinocyte-conditioned cell culture medium. We found increased activation of protein kinase B/Akt and mitogen-activated protein kinase pathways in protease-deficient cells, especially if treated with media conditioned by Ctsl-deficient keratinocytes. Similarly, the level of active GTP-Ras was increased in Ctsl-deficient epidermis. We conclude that Ctsl is critical for the termination of growth factor signaling in the endosomal/lysosomal compartment of keratinocytes and, therefore, functions as an anti-tumor protease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Abboud-Jarrous G, Atzmon R, Peretz T, Palermo C, Gadea BB, Joyce JA et al. (2008). Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J Biol Chem 283: 18167–18176.
Arbeit JM, Munger K, Howley PM, Hanahan D . (1994). Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 68: 4358–4368.
Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A et al. (2003). Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet 35: 252–257.
Bernard D, Mehul B, Thomas-Collignon A, Simonetti L, Remy V, Bernard MA et al. (2003). Analysis of proteins with caseinolytic activity in a human stratum corneum extract revealed a yet unidentified cysteine protease and identified the so-called ‘stratum corneum thiol protease’ as cathepsin L2. J Invest Dermatol 120: 592–600.
Bethel PA, Gerhardt S, Jones EV, Kenny PW, Karoutchi GI, Morley AD et al. (2009). Design of selective cathepsin inhibitors. Bioorg Med Chem Lett 19: 4622–4625.
Boudreau F, Lussier CR, Mongrain S, Darsigny M, Drouin JL, Doyon G et al. (2007). Loss of cathepsin L activity promotes claudin-1 overexpression and intestinal neoplasia. FASEB J 21: 3853–3865.
Brömme D . (2004). Cathepsin V. In: Barrett AJ, Rawlings ND, Woessner JF (eds). Handbook of Proteolytic Enzymes. Elsevier: London, pp 1107–1110.
Chan RJ, Feng GS . (2007). PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood 109: 862–867.
Cheng T, Hitomi K, van Vlijmen-Willems IM, de Jongh GJ, Yamamoto K, Nishi K et al. (2006). Cystatin M/E is a high affinity inhibitor of cathepsin V and cathepsin L by a reactive site that is distinct from the legumain-binding site. A novel clue for the role of cystatin M/E in epidermal cornification. J Biol Chem 281: 15893–15899.
Coussens LM, Hanahan D, Arbeit JM . (1996). Genetic predisposition and parameters of malignant progression in K14-HPV16 transgenic mice. Am J Pathol 149: 1899–1917.
Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z et al. (1999). Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev 13: 1382–1397.
Coussens LM, Tinkle CL, Hanahan D, Werb Z . (2000). MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103: 481–490.
Coussens LM, Fingleton B, Matrisian LM . (2002). Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295: 2387–2392.
Dano K, Romer J, Nielsen BS, Bjorn S, Pyke C, Rygaard J et al. (1999). Cancer invasion and tissue remodeling—cooperation of protease systems and cell types. Apmis 107: 120–127.
de Visser KE, Korets LV, Coussens LM . (2005). De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7: 411–423.
de Visser KE, Eichten A, Coussens LM . (2006). Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 6: 24–37.
DeClerck YA, Mercurio AM, Stack MS, Chapman HA, Zutter MM, Muschel RJ et al. (2004). Proteases, extracellular matrix, and cancer: a workshop of the path B study section. Am J Pathol 164: 1131–1139.
Felbor U, Dreier L, Bryant RA, Ploegh HL, Olsen BR, Mothes W . (2000). Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J 19: 1187–1194.
Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C et al. (2006). Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev 20: 543–556.
Gondi CS, Lakka SS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M et al. (2004). Adenovirus-mediated expression of antisense urokinase plasminogen activator receptor and antisense cathepsin B inhibits tumor growth, invasion, and angiogenesis in gliomas. Cancer Res 64: 4069–4077.
Goulet B, Sansregret L, Leduy L, Bogyo M, Weber E, Chauhan SS et al. (2007). Increased expression and activity of nuclear cathepsin L in cancer cells suggests a novel mechanism of cell transformation. Mol Cancer Res 5: 899–907.
Gutierrez-Fernandez A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S et al. (2008). Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res 68: 2755–2763.
Hagemann S, Gunther T, Dennemarker J, Lohmuller T, Bromme D, Schule R et al. (2004). The human cysteine protease cathepsin V can compensate for murine cathepsin L in mouse epidermis and hair follicles. Eur J Cell Biol 83: 775–780.
Haider AS, Peters SB, Kaporis H, Cardinale I, Fei J, Ott J et al. (2006). Genomic analysis defines a cancer-specific gene expression signature for human squamous cell carcinoma and distinguishes malignant hyperproliferation from benign hyperplasia. J Invest Dermatol 126: 869–881.
Jedeszko C, Sloane BF . (2004). Cysteine cathepsins in human cancer. Biol Chem 385: 1017–1027.
Kalluri R . (2003). Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3: 422–433.
Liotta LA, Kohn EC . (2001). The microenvironment of the tumour-host interface. Nature 411: 375–379.
Lopez-Otin C, Matrisian LM . (2007). Emerging roles of proteases in tumour suppression. Nat Rev Cancer 7: 800–808.
Martin MD, Matrisian LM . (2007). The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev 26: 717–724.
Mayerle J, Schnekenburger J, Kruger B, Kellermann J, Ruthenburger M, Weiss FU et al. (2005). Extracellular cleavage of E-cadherin by leukocyte elastase during acute experimental pancreatitis in rats. Gastroenterology 129: 1251–1267.
Mohamed MM, Sloane BF . (2006). Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer 6: 764–775.
Mueller MM, Fusenig NE . (2004). Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4: 839–849.
Navab R, Pedraza C, Fallavollita L, Wang N, Chevet E, Auguste P et al. (2008). Loss of responsiveness to IGF-I in cells with reduced cathepsin L expression levels. Oncogene 27: 4973–4985.
Neel BG, Gu H, Pao L . (2003). The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci 28: 284–293.
Nyberg P, Salo T, Kalluri R . (2008). Tumor microenvironment and angiogenesis. Front Biosci 13: 6537–6553.
Reinheckel T, Hagemann S, Dollwet-Mack S, Martinez E, Lohmuller T, Zlatkovic G et al. (2005). The lysosomal cysteine protease cathepsin L regulates keratinocyte proliferation by control of growth factor recycling. J Cell Sci 118: 3387–3395.
Roth W, Deussing J, Botchkarev VA, Pauly-Evers M, Saftig P, Hafner A et al. (2000). Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J 14: 2075–2086.
Schneider MR, Werner S, Paus R, Wolf E . (2008). Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol 173: 14–24.
Schubbert S, Shannon K, Bollag G . (2007). Hyperactive ras in developmental disorders and cancer. Nat Rev Cancer 7: 295–308.
Tobin DJ, Foitzik K, Reinheckel T, Mecklenburg L, Botchkarev VA, Peters C et al. (2002). The lysosomal protease cathepsin L is an important regulator of keratinocyte and melanocyte differentiation during hair follicle morphogenesis and cycling. Am J Pathol 160: 1807–1821.
Tolosa E, Li W, Yasuda Y, Wienhold W, Denzin LK, Lautwein A et al. (2003). Cathepsin V is involved in the degradation of invariant chain in human thymus and is overexpressed in myasthenia gravis. J Clin Invest 112: 517–526.
Urbich C, Heeschen C, Aicher A, Sasaki K, Bruhl T, Farhadi MR et al. (2005). Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med 11: 206–213.
Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J et al. (2006). Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res 66: 5242–5250.
Vasiljeva O, Reinheckel T, Peters C, Turk D, Turk V, Turk B . (2007). Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr Pharm Des 13: 387–403.
Vasiljeva O, Korovin M, Gajda M, Brodoefel H, Bojic L, Kruger A et al. (2008). Reduced tumour cell proliferation and delayed development of high-grade mammary carcinomas in cathepsin B-deficient mice. Oncogene 27: 4191–4199.
von Zastrow M, Sorkin A . (2007). Signaling on the endocytic pathway. Curr Opin Cell Biol 19: 436–445.
Walz M, Kellermann S, Bylaite M, Andree B, Ruther U, Paus R et al. (2007). Expression of the human cathepsin L inhibitor hurpin in mice: skin alterations and increased carcinogenesis. Exp Dermatol 16: 715–723.
Zheng X, Chou PM, Mirkin BL, Rebbaa A . (2004). Senescence-initiated reversal of drug resistance: specific role of cathepsin L. Cancer Res 64: 1773–1780.
Acknowledgements
We thank Ulrike Reif and Susanne Dollwet-Mack for excellent technical assistance and Dr Marie Follo for comments on the paper. The work was supported by a grant from the Deutsche Krebshilfe (Re106977) and in part by the Excellence Initiative of the German Federal and State Governments (EXC 294) and the European Union Framework Program (FP7 ‘MICROENVIMET’ No 201279).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Dennemärker, J., Lohmüller, T., Mayerle, J. et al. Deficiency for the cysteine protease cathepsin L promotes tumor progression in mouse epidermis. Oncogene 29, 1611–1621 (2010). https://doi.org/10.1038/onc.2009.466
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.466
Keywords
This article is cited by
-
Identification and characterization of the lamprey cathepsin genes
Immunogenetics (2019)
-
The role of proteases in epithelial-to-mesenchymal cell transitions in cancer
Cancer and Metastasis Reviews (2019)
-
Cysteine protease cathepsins in cardiovascular disease: from basic research to clinical trials
Nature Reviews Cardiology (2018)
-
Cathepsin L in tumor angiogenesis and its therapeutic intervention by the small molecule inhibitor KGP94
Clinical & Experimental Metastasis (2016)
-
The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses
Molecular Cancer (2015)