Abstract
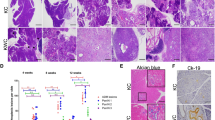
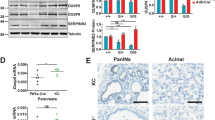
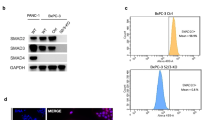
Mutations of SMAD4/DPC4 are found in about 60% of human invasive pancreatic ductal adenocarcinomas (PDACs); yet, the manner in which SMAD4 deficiency enhances tumorigenesis remains elusive. Using a Cre-LoxP approach, we generated a mutant mouse carrying a targeted deletion of Smad4 in the pancreas. We showed that the absence of Smad4 alone did not trigger pancreas tumor formation; however, it increased the expression of an inactivated form of Pten, suggesting a role of Pten in preventing Smad4–/– cells from undergoing malignancy. To investigate this, we disrupted both Pten and Smad4. We showed that Pten deficiency initiated widespread premalignant lesions, and a low tumor incidence that was significantly accelerated by Smad4-deficiency. The absence of Smad4 in a Pten-mutant background enhanced cell proliferation and triggered transdifferentiation from acinar, centroacinar and islet cells, accompanied by activation of Notch1 signaling. We showed that all tumors developed in the Smad4/Pten-mutant pancreas exhibited high levels of pAKT and mTOR, and that about 50 and 83% of human pancreatic cancers examined showed increased pAKT and pmTOR, respectively. Besides the similarity in gene expression, the pAKT and/or pmTOR-positive human PDACs and mouse pancreatic tumors also shared some histopathological similarities. These observations indicate that Smad4/Pten-mutant mice mimic the tumor progression of human pancreatic cancers that are driven by activation of the AKT–mTOR pathway, and uncovered a synergistic action of Smad4 and Pten in repressing pancreatic tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J et al. (2003). Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev 17: 3112–3126.
Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M . (1988). Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53: 549–554.
Altomare DA, Tanno S, De Rienzo A, Klein-Szanto AJ, Skele KL, Hoffman JP et al. (2003). Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem 88: 470–476.
Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA . (2004). The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene 23: 8571–8580.
Attri J, Srinivasan R, Majumdar S, Radotra BD, Wig J . (2005). Alterations of tumor suppressor gene p16INK4a in pancreatic ductal carcinoma. BMC Gastroenterol 5: 22.
Bardeesy N, DePinho RA . (2002). Pancreatic cancer biology and genetics. Nat Rev Cancer 2: 897–909.
Bardeesy N, Morgan J, Sinha M, Signoretti S, Srivastava S, Loda M et al. (2002). Obligate roles for p16(Ink4a) and p19(Arf)-p53 in the suppression of murine pancreatic neoplasia. Mol Cell Biol 22: 635–643.
Deramaudt T, Rustgi AK . (2005). Mutant KRAS in the initiation of pancreatic cancer. Biochim Biophys Acta 1756: 97–101.
Ebert MP, Fei G, Schandl L, Mawrin C, Dietzmann K, Herrera P et al. (2002). Reduced PTEN expression in the pancreas overexpressing transforming growth factor-beta 1. Br J Cancer 86: 257–262.
Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR et al. (2005). A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery 138: 618–628; discussion 628–630.
Friedl W, Kruse R, Uhlhaas S, Stolte M, Schartmann B, Keller KM et al. (1999). Frequent 4-bp deletion in exon 9 of the SMAD4/MADH4 gene in familial juvenile polyposis patients. Genes Chromosomes Cancer 25: 403–406.
Gannon M, Herrera PL, Wright CV . (2000). Mosaic Cre-mediated recombination in pancreas using the pdx-1 enhancer/promoter. Genesis 26: 143–144.
Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA et al. (2001). Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294: 2186–2189.
Gu G, Dubauskaite J, Melton DA . (2002). Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129: 2447–2457.
Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L et al. (2007). Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11: 291–302.
Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B et al. (2003). BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst 95: 214–221.
Hahn SA, Hoque AT, Moskaluk CA, da Costa LT, Schutte M, Rozenblum E et al. (1996a). Homozygous deletion map at 18q21.1 in pancreatic cancer. Cancer Res 56: 490–494.
Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E et al. (1996b). DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. [see comments]. Science 271: 350–353.
Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J . (2003). Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol 260: 426–437.
Heldin CH, Miyazono K, ten Dijke P . (1997). TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390: 465–471.
Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA . (2006). Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 20: 1218–1249.
Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA et al. (2003). Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4: 437–450.
Howe JR, Roth S, Ringold JC, Summers RW, Jarvinen HJ, Sistonen P et al. (1998). Mutations in the SMAD4/DPC4 gene in juvenile polyposis [see comments]. Science 280: 1086–1088.
Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP et al. (2006). Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res 66: 95–106.
Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S et al. (2006). Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev 20: 3147–3160.
Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM et al. (2007). Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell 11: 229–243.
Jarriault S, Le Bail O, Hirsinger E, Pourquie O, Logeat F, Strong CF et al. (1998). Delta-1 activation of notch-1 signaling results in HES-1 transactivation. Mol Cell Biol 18: 7423–7431.
Kang YK, Kim WH, Jang JJ . (2002). Expression of G1-S modulators (p53, p16, p27, cyclin D1, Rb) and Smad4/Dpc4 in intrahepatic cholangiocarcinoma. Hum Pathol 33: 877–883.
Kim BG, Li C, Qiao W, Mamura M, Kasperczak B, Anver M et al. (2006). Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature 441: 1015–1019.
Li W, Qiao W, Chen L, Xu X, Yang X, Li D et al. (2003). Squamous cell carcinoma and mammary abscess formation through squamous metaplasia in Smad4/Dpc4 conditional knockout mice. Development 130: 6143–6153.
Maitra A, Hruban RH . (2008). Pancreatic cancer. Annu Rev Pathol 3: 157–188.
Maitra A, Kern SE, Hruban RH . (2006). Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol 20: 211–226.
Massague J . (1998). TGF-beta signal transduction. Ann Rev Biochem 67: 753–791.
Michl P, Downward J . (2005). Mechanisms of disease: PI3K/AKT signaling in gastrointestinal cancers. Z Gastroenterol 43: 1133–1139.
Murtaugh LC, Stanger BZ, Kwan KM, Melton DA . (2003). Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA 100: 14920–14925.
Peng B, Fleming JB, Breslin T, Grau AM, Fojioka S, Abbruzzese JL et al. (2002). Suppression of tumorigenesis and induction of p15(ink4b) by Smad4/DPC4 in human pancreatic cancer cells. Clin Cancer Res 8: 3628–3638.
Qiao W, Li AG, Owens P, Xu X, Wang XJ, Deng CX . (2006). Hair follicle defects and squamous cell carcinoma formation in Smad4 conditional knockout mouse skin. Oncogene 25: 207–217.
Roy M, Pear WS, Aster JC . (2007). The multifaceted role of Notch in cancer. Curr Opin Genet Dev 17: 52–59.
Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M et al. (1997). Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res 57: 1731–1734.
Sawey ET, Johnson JA, Crawford HC . (2007). Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc Natl Acad Sci USA 104: 19327–19332.
Schroeter EH, Kisslinger JA, Kopan R . (1998). Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393: 382–386.
Semba S, Moriya T, Kimura W, Yamakawa M . (2003). Phosphorylated Akt/PKB controls cell growth and apoptosis in intraductal papillary-mucinous tumor and invasive ductal adenocarcinoma of the pancreas. Pancreas 26: 250–257.
Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A et al. (1998). The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev 12: 107–119.
Soriano P . (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71.
Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y et al. (2005). Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell 8: 185–195.
Suto T, Sugai T, Habano W, Uesugi N, Kanno S, Saito K et al. (2002). Allelotype analysis of the PTEN, Smad4 and DCC genes in biliary tract cancer. Anticancer Res 22: 1529–1536.
Takaku K, Miyoshi H, Matsunaga A, Oshima M, Sasaki N, Taketo MM . (1999). Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res 59: 6113–6117.
Tamura G, Sakata K, Nishizuka S, Maesawa C, Suzuki Y, Terashima M et al. (1996). Allelotype of adenoma and differentiated adenocarcinoma of the stomach. J Pathol 180: 371–377.
Teng Y, Sun AN, Pan XC, Yang G, Yang LL, Wang MR et al. (2006). Synergistic function of Smad4 and PTEN in suppressing forestomach squamous cell carcinoma in the mouse. Cancer Res 66: 6972–6981.
Warshaw AL, Fernandez-del Castillo C . (1992). Pancreatic carcinoma. N Engl J Med 326: 455–465.
Weinstein M, Deng CX . (2006). Genetic disruptions within the murine genome reveal the numerous roles of the Smad gene family in development, disease, and cancer, vol. 5. Springer: Dordrecht, 151–176pp.
Weinstein M, Yang X, Deng C . (2000). Functions of mammalian Smad genes as revealed by targeted gene disruption in mice. Cytokine Growth Factor Rev 11: 49–58.
Xu X, Brodie SG, Yang X, Im YH, Parks WT, Chen L et al. (2000). Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene 19: 1868–1874.
Xu X, Kobayashi S, Qiao W, Li C, Xiao C, Radaeva S et al. (2006). Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. J Clin Invest 116: 1843–1852.
Yachida S, Iacobuzio-Donahue CA . (2009). The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med 133: 413–422.
Yang L, Mao C, Teng Y, Li W, Zhang J, Cheng X et al. (2005). Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res 65: 8671–8678.
Yang X, Li C, Herrera PL, Deng CX . (2002). Generation of Smad4/Dpc4 conditional knockout mice. Genesis 32: 80–81.
Yang X, Li C, Xu X, Deng C . (1998). The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci USA 95: 3667–3672.
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK et al. (2002). Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 236: 355–366; discussion 366–358.
Acknowledgements
We thank Dr Douglas Melton for providing Pdx-Cre mice; Dr Hong Wu for Pten conditional mutant mice; and members of the Deng lab for critically reading the paper. This research was supported by the Intramural Research Program of the National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Xu, X., Ehdaie, B., Ohara, N. et al. Synergistic action of Smad4 and Pten in suppressing pancreatic ductal adenocarcinoma formation in mice. Oncogene 29, 674–686 (2010). https://doi.org/10.1038/onc.2009.375
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.375
Keywords
This article is cited by
-
Gastric Lgr5+ stem cells are the cellular origin of invasive intestinal-type gastric cancer in mice
Cell Research (2016)
-
Smad4 loss promotes lung cancer formation but increases sensitivity to DNA topoisomerase inhibitors
Oncogene (2016)
-
Inflammation and pancreatic cancer: molecular and functional interactions between S100A8, S100A9, NT-S100A8 and TGFβ1
Cell Communication and Signaling (2014)
-
Association of microRNA-21 expression with its targets, PDCD4 and TIMP3, in pancreatic ductal adenocarcinoma
Modern Pathology (2012)
-
Role of Smads in TGFβ signaling
Cell and Tissue Research (2012)