Abstract
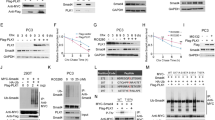
SHP-1, a haematopoietic cell-specific tyrosine phosphatase, is also expressed in human prostate. In this study, we report that SHP-1 depletion in PC-3 cells induced by small interfering RNAs causes G1 phase cell-cycle arrest accompanied by changes in some components of the cell-cycle machinery. SHP-1 knockdown increases p27Kip1 (p27) protein stability, its nuclear localization and p27 gene transcription. These effects could be mediated by PI3K-AKT pathway as SHP-1 interacts with PI3K regulating its activity and p110 catalytic subunit phosphorylation. The increase in p27 protein stability could also because of reduced cyclin-dependent kinase (CDK2) activity. SHP-1 knockdown decreases the CDK6 levels, inducing retinoblastoma protein hypophosphorylation, downregulation of cyclin E and thereby a decrease in the CDK2 activity. However, the codepletion of SHP-1 and p27 does not produce re-entry into the cycle, implying that p27 is not required to maintain cell-cycle arrest induced by SHP-1 depletion. The maintenance of the PC-3 cell anti-proliferative response after p27 loss could be because of mislocalization of CDK2 induced by SHP-1 knockdown. This study shows that SHP-1 depletion promotes cell-cycle arrest by modulating the activity of cell-cycle regulators and suggests that SHP-1 may be required for the proper functioning of events governing cell-cycle progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M et al. (1997). Role of translocation in the activation and function of protein kinase B. J Biol Chem 272: 31515–31524.
Besson A, Assoian RK, Roberts JM . (2004). Regulation of the cytoskeleton: an oncogenic function for CDK inhibitors? Nat Rev Cancer 4: 948–955.
Besson A, Dowdy SF, Roberts JM . (2008). CDK inhibitors: cell cycle regulators and beyond. Dev Cell 14: 159–169.
Boehm M, Yoshimoto T, Crook MF, Nallamshetty S, True A, Nabel GJ et al. (2002). A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J 21: 3390–3401.
Caron D, Savard PE, Doillon CJ, Olivier M, Shink E, Lussier JG et al. (2008). Protein tyrosine phosphatase inhibition induces anti-tumor activity: evidence of Cdk2/p27 kip1 and Cdk2/SHP-1 complex formation in human ovarian cancer cells. Cancer Lett 262: 265–275.
Craggs G, Kellie S . (2001). A functional nuclear localization sequence in the C-terminal domain of SHP-1. J Biol Chem 276: 23719–23725.
Cuevas BD, Lu Y, Mao M, Zhang J, LaPushin R, Siminovitch K et al. (2001). Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J Biol Chem 276: 27455–27461.
Chen HE, Chang S, Trub T, Neel BG . (1996). Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol 16: 3685–3697.
Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S et al. (2007). p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 128: 281–294.
Fujita N, Sato S, Katayama K, Tsuruo T . (2002). Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem 277: 28706–28713.
Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB et al. (2007). Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128: 269–280.
Harbour JW, Dean DC . (2000). The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev 14: 2393–2409.
Hengst L, Reed SI . (1996). Translational control of p27Kip1 accumulation during the cell cycle. Science 271: 1861–1864.
Hoffmann I, Draetta G, Karsenti E . (1994). Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J 13: 4302–4310.
Inoue T, Kamiyama J, Sakai T . (1999). Sp1 and NF-Y synergistically mediate the effect of vitamin D(3) in the p27(Kip1) gene promoter that lacks vitamin D response elements. J Biol Chem 274: 32309–32317.
Larionov A, Krause A, Miller W . (2005). A standard curve based method for relative real time PCR data processing. BMC Bioinformatics 6: 62.
Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K et al. (2002). PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 8: 1153–1160.
Malumbres M, Barbacid M . (2009). Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9: 153–166.
Martin-Orozco RM, Almaraz-Pro C, Rodriguez-Ubreva FJ, Cortes MA, Ropero S, Colomer R et al. (2007). EGF prevents the neuroendocrine differentiation of LNCaP cells induced by serum deprivation: the modulator role of PI3K/Akt. Neoplasia 9: 614–624.
Medema RH, Kops GJ, Bos JL, Burgering BM . (2000). AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404: 782–787.
Moore JD, Yang J, Truant R, Kornbluth S . (1999). Nuclear import of Cdk/cyclin complexes: identification of distinct mechanisms for import of Cdk2/cyclin E and Cdc2/cyclin B1. J Cell Biol 144: 213–224.
Nakagawa H, Mutoh T, Kumano T, Kuriyama M . (2001). Tyrosine phosphorylation of the catalytic subunit p110 of phosphatidylinositol-3 kinase induced by HMG-CoA reductase inhibitor inhibits its kinase activity in L6 myoblasts. FEBS Lett 508: 53–56.
Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V et al. (1995). Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269: 682–685.
Pages P, Benali N, Saint-Laurent N, Esteve JP, Schally AV, Tkaczuk J et al. (1999). sst2 somatostatin receptor mediates cell cycle arrest and induction of p27(Kip1). Evidence for the role of SHP-1. J Biol Chem 274: 15186–15193.
Pani G, Fischer KD, Mlinaric-Rascan I, Siminovitch KA . (1996). Signaling capacity of the T cell antigen receptor is negatively regulated by the PTP1C tyrosine phosphatase. J Exp Med 184: 839–852.
Pani G, Kozlowski M, Cambier JC, Mills GB, Siminovitch KA . (1995). Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor-associated protein involved in the regulation of B cell signaling. J Exp Med 181: 2077–2084.
Ram PA, Waxman DJ . (1997). Interaction of growth hormone-activated STATs with SH2-containing phosphotyrosine phosphatase SHP-1 and nuclear JAK2 tyrosine kinase. J Biol Chem 272: 17694–17702.
Sebastian B, Kakizuka A, Hunter T . (1993). Cdc25M2 activation of cyclin-dependent kinases by dephosphorylation of threonine-14 and tyrosine-15. Proc Natl Acad Sci USA 90: 3521–3524.
Seo DW, Li H, Qu CK, Oh J, Kim YS, Diaz T et al. (2006). Shp-1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. J Biol Chem 281: 3711–3721.
Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE . (1997). Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev 11: 1464–1478.
Sherr CJ . (1994). G1 phase progression: cycling on cue. Cell 79: 551–555.
Sherr CJ, Roberts JM . (1999). CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13: 1501–1512.
Simoneau M, Boulanger J, Coulombe G, Renaud MA, Duchesne C, Rivard N . (2008). Activation of Cdk2 stimulates proteasome-dependent truncation of tyrosine phosphatase SHP-1 in human proliferating intestinal epithelial cells. J Biol Chem 283: 25544–25556.
Slingerland J, Pagano M . (2000). Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol 183: 10–17.
Somani AK, Bignon JS, Mills GB, Siminovitch KA, Branch DR . (1997). Src kinase activity is regulated by the SHP-1 protein-tyrosine phosphatase. J Biol Chem 272: 21113–21119.
Su L, Zhao Z, Bouchard P, Banville D, Fischer EH, Krebs EG et al. (1996). Positive effect of overexpressed protein-tyrosine phosphatase PTP1C on mitogen-activated signaling in 293 cells. J Biol Chem 271: 10385–10390.
Tenev T, Bohmer SA, Kaufmann R, Frese S, Bittorf T, Beckers T et al. (2000). Perinuclear localization of the protein-tyrosine phosphatase SHP-1 and inhibition of epidermal growth factor-stimulated STAT1/3 activation in A431 cells. Eur J Cell Biol 79: 261–271.
Valencia AM, Oliva JL, Bodega G, Chiloeches A, Lopez-Ruiz P, Prieto JC et al. (1997). Identification of a protein-tyrosine phosphatase (SHP1) different from that associated with acid phosphatase in rat prostate. FEBS Lett 406: 42–48.
Yi T, Ihle JN . (1993). Association of hematopoietic cell phosphatase with c-Kit after stimulation with c-Kit ligand. Mol Cell Biol 13: 3350–3358.
Yi T, Mui AL, Krystal G, Ihle JN . (1993). Hematopoietic cell phosphatase associates with the interleukin-3 (IL-3) receptor beta chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Mol Cell Biol 13: 7577–7586.
You M, Zhao Z . (1997). Positive effects of SH2 domain-containing tyrosine phosphatase SHP-1 on epidermal growth factor- and interferon-gamma-stimulated activation of STAT transcription factors in HeLa cells. J Biol Chem 272: 23376–23381.
Yu Z, Su L, Hoglinger O, Jaramillo ML, Banville D, Shen SH . (1998). SHP-1 associates with both platelet-derived growth factor receptor and the p85 subunit of phosphatidylinositol 3-kinase. J Biol Chem 273: 3687–3694.
Zapata PD, Ropero RM, Valencia AM, Buscail L, Lopez JI, Martin-Orozco RM et al. (2002). Autocrine regulation of human prostate carcinoma cell proliferation by somatostatin through the modulation of the SH2 domain containing protein tyrosine phosphatase (SHP)-1. J Clin Endocrinol Metab 87: 915–926.
Zarkowska T, Mittnacht S . (1997). Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem 272: 12738–12746.
Acknowledgements
We are very grateful to Dr T Sakai (Department of Molecular-Targeting Cancer Prevention, Kyoto Prefectural University of Medicine, Kyoto, Japan) for providing human p27 promoter containing luciferase reporter construct (p27PF) and control empty vector and also to Dr FD Böhmer (Institute of Molecular Cell Biology, Medical Faculty, Friedrich Schiller University, Jena, Germany) for providing GST–SHP-1 wild type. This work was supported by Consejería de Sanidad de Castilla-La Mancha grant (04077-00); Fundación de Investigación Mutua Madrileña grant; Instituto de Salud Carlos III grant (PI060109); FPI fellowship, Comunidad de Madrid (FJ Rodriguez-Ubreva); Fundación Carolina and FPI fellowship Consejería de Educación de Castilla-La Mancha (AE Cariaga-Martinez).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Rodríguez-Ubreva, F., Cariaga-Martinez, A., Cortés, M. et al. Knockdown of protein tyrosine phosphatase SHP-1 inhibits G1/S progression in prostate cancer cells through the regulation of components of the cell-cycle machinery. Oncogene 29, 345–355 (2010). https://doi.org/10.1038/onc.2009.329
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.329
Keywords
This article is cited by
-
PDZK1 inhibits the development and progression of renal cell carcinoma by suppression of SHP-1 phosphorylation
Oncogene (2017)
-
The regulatory roles of phosphatases in cancer
Oncogene (2014)
-
PTPN6 expression is epigenetically regulated and influences survival and response to chemotherapy in high-grade gliomas
Tumor Biology (2014)
-
SHP1-mediated cell cycle redistribution inhibits radiosensitivity of non-small cell lung cancer
Radiation Oncology (2013)
-
Effects of low-level laser irradiation on mesenchymal stem cell proliferation: a microarray analysis
Lasers in Medical Science (2012)