Abstract
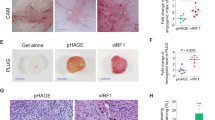
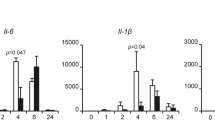
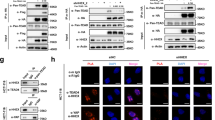
The mitotic checkpoint gene CHFR (checkpoint with forkhead and ring finger domains) is silenced in various human cancers by promoter hypermethylation, suggesting that CHFR is a tumor suppressor. Here, we show that CHFR functions as a negative regulator of the nuclear factor-κB (NF-κB) pathway. Expression of CHFR inhibited NF-κB reporter activity, whereas knockdown of CHFR activated reporter activity. These activities are independent of its RING finger domain. Furthermore, we found that CHFR physically interacts with p65 in cells. Electrophoretic mobility shift assays (EMSAs) and ELISA-based NF-κB-binding assays showed that CHFR negatively regulated transcriptional activity of p65. In addition, our data show that interleukin (IL)-8 is significantly downregulated by CHFR, and that the migration of human endothelial cells is suppressed in culture medium conditioned from CHFR-expressing cancer cells. Using a xenograft model, we show that neovascularization is suppressed by adenovirus-mediated transfer of CHFR. These results indicate that expression of CHFR markedly reduces the expression of IL-8 through the inhibition of NF-κB. As the NF-κB signaling pathway plays a critical role in the development and progression of cancer, our findings show the functional relationship between epigenetic alteration and inflammation/angiogenesis in human cancer cells, thereby showing several potential targets for therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y et al. (1990). A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J 9: 1897–1906.
Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF et al. (2007). Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell 129: 1065–1079.
Cabannes E, Khan G, Aillet F, Jarrett RF, Hay RT . (1999). Mutations in the IkBa gene in Hodgkin's disease suggest a tumour suppressor role for IkappaBalpha. Oncogene 18: 3063–3070.
Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ . (1998). Increasing complexity of Ras signaling. Oncogene 17: 1395–1413.
Cude K, Wang Y, Choi HJ, Hsuan SL, Zhang H, Wang CY et al. (2007). Regulation of the G2–M cell cycle progression by the ERK5–NFkappaB signaling pathway. J Cell Biol 177: 253–264.
Cuenca RE, Azizkhan RG, Haskill S . (1992). Characterization of GRO alpha, beta and gamma expression in human colonic tumours: potential significance of cytokine involvement. Surg Oncol 1: 323–329.
Daniels MJ, Marson A, Venkitaraman AR . (2004). PML bodies control the nuclear dynamics and function of the CHFR mitotic checkpoint protein. Nat Struct Mol Biol 11: 1114–1121.
Durocher D, Jackson SP . (2002). The FHA domain. FEBS Lett 513: 58–66.
Emmerich F, Theurich S, Hummel M, Haeffker A, Vry MS, Dohner K et al. (2003). Inactivating I kappa B epsilon mutations in Hodgkin/Reed–Sternberg cells. J Pathol 201: 413–420.
Fukuda T, Kondo Y, Nakagama H . (2008). The anti-proliferative effects of the CHFR depend on the forkhead associated domain, but not E3 ligase activity mediated by ring finger domain. PLoS ONE 3: e1776.
Hofmann K, Bucher P . (1995). The FHA domain: a putative nuclear signalling domain found in protein kinases and transcription factors. Trends Biochem Sci 20: 347–349.
Jackson PK, Eldridge AG, Freed E, Furstenthal L, Hsu JY, Kaiser BK et al. (2000). The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol 10: 429–439.
Kang D, Chen J, Wong J, Fang G . (2002). The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J Cell Biol 156: 249–259.
Kang D, Wong J, Fang G . (2004). A Xenopus cell-free system for analysis of the Chfr ubiquitin ligase involved in control of mitotic entry. Methods Mol Biol 280: 229–243.
Karin M, Cao Y, Greten FR, Li ZW . (2002). NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2: 301–310.
Kawai N, Takahashi H, Nishida H, Yokosawa H . (2005). Regulation of NF-kappaB/Rel by IkappaB is essential for ascidian notochord formation. Dev Biol 277: 80–91.
Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ et al. (2007). Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell 12: 131–144.
Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM et al. (1992). Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258: 1798–1801.
Kontermann RE, Munkel S, Neumeyer J, Muller D, Branschadel M, Scheurich P et al. (2008). A humanized tumor necrosis factor receptor 1 (TNFR1)-specific antagonistic antibody for selective inhibition of tumor necrosis factor (TNF) action. J Immunother 31: 225–234.
Kunsch C, Rosen CA . (1993). NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol 13: 6137–6146.
Lee KH, Bae SH, Lee JL, Hyun MS, Kim SH, Song SK et al. (2004). Relationship between urokinase-type plasminogen receptor, interleukin-8 gene expression and clinicopathological features in gastric cancer. Oncology 66: 210–217.
Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW et al. (2008). Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 319: 1676–1679.
Libermann TA, Baltimore D . (1990). Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 10: 2327–2334.
Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T et al. (1993). Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA 90: 10193–10197.
Mumm JB, Oft M . (2008). Cytokine-based transformation of immune surveillance into tumor-promoting inflammation. Oncogene 27: 5913–5919.
Nishikawa N, Toyota M, Suzuki H, Honma T, Fujikane T, Ohmura T et al. (2007). Gene amplification and overexpression of PRDM14 in breast cancers. Cancer Res 67: 9649–9657.
Nurnberg W, Tobias D, Otto F, Henz BM, Schadendorf D . (1999). Expression of interleukin-8 detected by in situ hybridization correlates with worse prognosis in primary cutaneous melanoma. J Pathol 189: 546–551.
Ogi K, Toyota M, Mita H, Satoh A, Kashima L, Sasaki Y et al. (2005). Small interfering RNA-induced CHFR silencing sensitizes oral squamous cell cancer cells to microtubule inhibitors. Cancer Biol Ther 4: 773–780.
Privette LM, Gonzalez ME, Ding L, Kleer CG, Petty EM . (2007). Altered expression of the early mitotic checkpoint protein, CHFR, in breast cancers: implications for tumor suppression. Cancer Res 67: 6064–6074.
Privette LM, Petty EM . (2008). CHFR: a novel mitotic checkpoint protein and regulator of tumorigenesis. Transl Oncol 1: 57–64.
Rakitina TV, Vasilevskaya IA, O'Dwyer PJ . (2003). Additive interaction of oxaliplatin and 17-allylamino-17-demethoxygeldanamycin in colon cancer cell lines results from inhibition of nuclear factor kappaB signaling. Cancer Res 63: 8600–8605.
Rayet B, Gelinas C . (1999). Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18: 6938–6947.
Rosette C, Karin M . (1995). Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappa B. J Cell Biol 128: 1111–1119.
Sasaki Y, Ishida S, Morimoto I, Yamashita T, Kojima T, Kihara C et al. (2002). The p53 family member genes are involved in the Notch signal pathway. J Biol Chem 277: 719–724.
Sasaki Y, Morimoto I, Ishida S, Yamashita T, Imai K, Tokino T . (2001). Adenovirus-mediated transfer of the p53 family genes, p73 and p51/p63 induces cell cycle arrest and apoptosis in colorectal cancer cell lines: potential application to gene therapy of colorectal cancer. Gene Ther 8: 1401–1408.
Sathe SS, Sizemore N, Li X, Vithalani K, Commane M, Swiatkowski SM et al. (2004). Mutant human cells with constitutive activation of NF-kappaB. Proc Natl Acad Sci USA 101: 192–197.
Satoh A, Toyota M, Itoh F, Sasaki Y, Suzuki H, Ogi K et al. (2003). Epigenetic inactivation of CHFR and sensitivity to microtubule inhibitors in gastric cancer. Cancer Res 63: 8606–8613.
Scolnick DM, Halazonetis TD . (2000). Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature 406: 430–435.
Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD et al. (1994). Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med 179: 1409–1415.
Starczynowski DT, Trautmann H, Pott C, Harder L, Arnold N, Africa JA et al. (2007). Mutation of an IKK phosphorylation site within the transactivation domain of REL in two patients with B-cell lymphoma enhances REL's in vitro transforming activity. Oncogene 26: 2685–2694.
Tomita S, Fujita T, Kirino Y, Suzuki T . (2000). PDZ domain-dependent suppression of NF-kappaB/p65-induced Abeta42 production by a neuron-specific X11-like protein. J Biol Chem 275: 13056–13060.
Toyota M, Sasaki Y, Satoh A, Ogi K, Kikuchi T, Suzuki H et al. (2003). Epigenetic inactivation of CHFR in human tumors. Proc Natl Acad Sci USA 100: 7818–7823.
Wan F, Anderson DE, Barnitz RA, Snow A, Bidere N, Zheng L et al. (2007). Ribosomal protein S3: a KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell 131: 927–939.
Williams JL, Ji P, Ouyang N, Liu X, Rigas B . (2008). NO-donating aspirin inhibits the activation of NF-kappaB in human cancer cell lines and Min mice. Carcinogenesis 29: 390–397.
Wu WS, Xu ZX, Hittelman WN, Salomoni P, Pandolfi PP, Chang KS . (2003). Promyelocytic leukemia protein sensitizes tumor necrosis factor alpha-induced apoptosis by inhibiting the NF-kappaB survival pathway. J Biol Chem 278: 12294–12304.
Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K . (1992). Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. J Biol Chem 267: 22506–22511.
Yu X, Minter-Dykhouse K, Malureanu L, Zhao WM, Zhang D, Merkle CJ et al. (2005). Chfr is required for tumor suppression and Aurora A regulation. Nat Genet 37: 401–406.
Yuan A, Chen JJ, Yao PL, Yang PC . (2005). The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci 10: 853–865.
Acknowledgements
This research was supported by a grant to TT (KAKENHI (20390519)) from the Grants-in-Aid for Scientific Research (B) program of the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT). We thank Dr Toshiharu Suzuki (Hokkaido University, Japan) for the pcDNA3-Flag-p65 construct.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Rights and permissions
About this article
Cite this article
Kashima, L., Toyota, M., Mita, H. et al. CHFR, a potential tumor suppressor, downregulates interleukin-8 through the inhibition of NF-κB. Oncogene 28, 2643–2653 (2009). https://doi.org/10.1038/onc.2009.123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.123
Keywords
This article is cited by
-
CHFR regulates chemoresistance in triple-negative breast cancer through destabilizing ZEB1
Cell Death & Disease (2021)
-
Emerging evidence for CHFR as a cancer biomarker: from tumor biology to precision medicine
Cancer and Metastasis Reviews (2013)
-
The diverse and complex roles of NF-κB subunits in cancer
Nature Reviews Cancer (2012)
-
The nuclear signaling of NF-κB: current knowledge, new insights, and future perspectives
Cell Research (2010)