Abstract
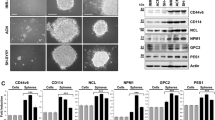
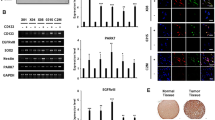
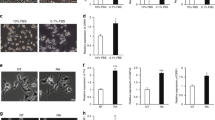
The biological and clinical heterogeneity of neuroblastoma is closely associated with signaling pathways that control cellular characteristics such as proliferation, survival and differentiation. The Shc family of docking proteins is important in these pathways by mediating cellular signaling. In this study, we analysed the expression levels of ShcA and ShcC proteins in 46 neuroblastoma samples and showed that a significantly higher level of ShcC protein is observed in neuroblastomas with poor prognostic factors such as advanced stage and MYCN amplification (P<0.005), whereas the expression level of ShcA showed no significant association with these factors. Using TNB1 cells that express a high level of ShcC protein, it was demonstrated that knockdown of ShcC by RNAi caused elevation in the phosphorylation of ShcA, which resulted in sustained extracellular signal-regulated kinase activation and neurite outgrowth. The neurites induced by ShcC knockdown expressed several markers of neuronal differentiation suggesting that the expression of ShcC potentially has a function in inhibiting the differentiation of neuroblastoma cells. In addition, marked suppression of in vivo tumorigenicity of TNB1 cells in nude mice was observed by stable knockdown of ShcC protein. These findings indicate that ShcC is a therapeutic target that might induce differentiation in the aggressive type of neuroblastomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Berwanger B, Hartmann O, Bergmann E, Bernard S, Nielsen D, Krause M et al. (2002). Loss of a FYN-regulated differentiation and growth arrest pathway in advanced stage neuroblastoma. Cancer Cell 2: 377–386.
Brodeur GM, Nakagawara A . (1992). Molecular basis of clinical heterogeneity in neuroblastoma. Am J Pediatr Hematol Oncol 14: 111–116.
Brouns MR, Matheson SF, Hu KQ, Delalle I, Caviness VS, Silver J et al. (2000). The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development 127: 4891–4903.
Brouns MR, Matheson SF, Settleman J . (2001). p190 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation. Nat Cell Biol 3: 361–367.
Dhillon AS, Meikle S, Peyssonnaux C, Grindlay J, Kaiser C, Steen H et al. (2003). A Raf-1 mutant that dissociates MEK/extracellular signal-regulated kinase activation from malignant transformation and differentiation but not proliferation. Mol Cell Biol 23: 1983–1993.
Giudici AM, Sher E, Pelagi M, Clementi F, Zanini A . (1992). Immunolocalization of secretogranin II, chromogranin A, and chromogranin B in differentiating human neuroblastoma cells. Eur J Cell Biol 58: 383–389.
Hecht M, Papoutsi M, Tran HD, Wilting J, Schweigerer L . (2004). Hepatocyte growth factor/c-Met signaling promotes the progression of experimental human neuroblastomas. Cancer Res 64: 6109–6118.
Hecker TP, Grammer JR, Gillespie GY, Stewart Jr J, Gladson CL . (2002). Focal adhesion kinase enhances signaling through the Shc/extracellular signal-regulated kinase pathway in anaplastic astrocytoma tumor biopsy samples. Cancer Res 62: 2699–2707.
Hinsby AM, Lundfald L, Ditlevsen DK, Korshunova I, Juhl L, Meakin SO et al. (2004). ShcA regulates neurite outgrowth stimulated by neural cell adhesion molecule but not by fibroblast growth factor 2: evidence for a distinct fibroblast growth factor receptor response to neural cell adhesion molecule activation. J Neurochem 91: 694–703.
Iwamoto T, Taniguchi M, Wajjwalku W, Nakashima I, Takahashi M . (1993). Neuroblastoma in a transgenic mouse carrying a metallothionein/ret fusion gene. Br J Cancer 67: 504–507.
Leevers SJ, Paterson HF, Marshall CJ . (1994). Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 369: 411–414.
Liu HY, Meakin SO . (2002). ShcB and ShcC activation by the Trk family of receptor tyrosine kinases. J Biol Chem 277: 26046–26056.
Magrassi L, Conti L, Lanterna A, Zuccato C, Marchionni M, Cassini P et al. (2005). Shc3 affects human high-grade astrocytomas survival. Oncogene 24: 5198–5206.
Marshall GM, Peaston AE, Hocker JE, Smith SA, Hansford LM, Tobias V et al. (1997). Expression of multiple endocrine neoplasia 2B RET in neuroblastoma cells alters cell adhesion in vitro, enhances metastatic behavior in vivo, and activates Jun kinase. Cancer Res 57: 5399–5405.
Miyake I, Hakomori Y, Misu Y, Nakadate H, Matsuura N, Sakamoto M et al. (2005). Domain-specific function of ShcC docking protein in neuroblastoma cells. Oncogene 24: 3206–3215.
Miyake I, Hakomori Y, Shinohara A, Gamou T, Saito M, Iwamatsu A et al. (2002). Activation of anaplastic lymphoma kinase is responsible for hyperphosphorylation of ShcC in neuroblastoma cell lines. Oncogene 21: 5823–5834.
Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L et al. (1998). Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J 17: 6622–6632.
Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM . (1993). Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med 328: 847–854.
Nakagawara A, Brodeur GM . (1997). Role of neurotrophins and their receptors in human neuroblastomas: a primary culture study. Eur J Cancer 33: 2050–2053.
Nakamura T, Komiya M, Gotoh N, Koizumi S, Shibuya M, Mori N . (2002). Discrimination between phosphotyrosine-mediated signaling properties of conventional and neuronal Shc adapter molecules. Oncogene 21: 22–31.
Nakamura T, Muraoka S, Sanokawa R, Mori N . (1998). N-Shc and Sck, two neuronally expressed Shc adapter homologs. Their differential regional expression in the brain and roles in neurotrophin and Src signaling. J Biol Chem 273: 6960–6967.
Nakamura T, Sanokawa R, Sasaki Y, Ayusawa D, Oishi M, Mori N . (1996). N-Shc: a neural-specific adapter molecule that mediates signaling from neurotrophin/Trk to Ras/MAPK pathway. Oncogene 13: 1111–1121.
O'Bryan JP, Lambert QT, Der CJ . (1998). The src homology 2 and phosphotyrosine binding domains of the ShcC adaptor protein function as inhibitors of mitogenic signaling by the epidermal growth factor receptor. J Biol Chem 273: 20431–20437.
O'Bryan JP, Songyang Z, Cantley L, Der CJ, Pawson T . (1996). A mammalian adaptor protein with conserved Src homology 2 and phosphotyrosine-binding domains is related to Shc and is specifically expressed in the brain. Proc Natl Acad Sci USA 93: 2729–2734.
Ohira M, Morohashi A, Inuzuka H, Shishikura T, Kawamoto T, Kageyama H et al. (2003). Expression profiling and characterization of 4200 genes cloned from primary neuroblastomas: identification of 305 genes differentially expressed between favorable and unfavorable subsets. Oncogene 22: 5525–5536.
Opel D, Poremba C, Simon T, Debatin KM, Fulda S . (2007). Activation of Akt predicts poor outcome in neuroblastoma. Cancer Res 67: 735–745.
Osajima-Hakomori Y, Miyake I, Ohira M, Nakagawara A, Nakagawa A, Sakai R . (2005). Biological role of anaplastic lymphoma kinase in neuroblastoma. Am J Pathol 167: 213–222.
Pelicci G, Dente L, De Giuseppe A, Verducci-Galletti B, Giuli S, Mele S et al. (1996). A family of Shc related proteins with conserved PTB, CH1 and SH2 regions. Oncogene 13: 633–641.
Qui MS, Green SH . (1992). PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron 9: 705–717.
Ravichandran KS . (2001). Signaling via Shc family adapter proteins. Oncogene 20: 6322–6330.
Sakai R, Henderson JT, O'Bryan JP, Elia AJ, Saxton TM, Pawson T . (2000). The mammalian ShcB and ShcC phosphotyrosine docking proteins function in the maturation of sensory and sympathetic neurons. Neuron 28: 819–833.
Schwab M, Westermann F, Hero B, Berthold F . (2003). Neuroblastoma: biology and molecular and chromosomal pathology. Lancet Oncol 4: 472–480.
Stokoe D, Macdonald SG, Cadwallader K, Symons M, Hancock JF . (1994). Activation of Raf as a result of recruitment to the plasma membrane. Science 264: 1463–1467.
Terui E, Matsunaga T, Yoshida H, Kouchi K, Kuroda H, Hishiki T et al. (2005). Shc family expression in neuroblastoma: high expression of shcC is associated with a poor prognosis in advanced neuroblastoma. Clin Cancer Res 11: 3280–3287.
Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG . (1996). The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87: 733–743.
Wary KK, Mariotti A, Zurzolo C, Giancotti FG . (1998). A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94: 625–634.
Wellbrock C, Weisser C, Geissinger E, Troppmair J, Schartl M . (2002). Activation of p59(Fyn) leads to melanocyte dedifferentiation by influencing MKP-1-regulated mitogen-activated protein kinase signaling. J Biol Chem 277: 6443–6454.
Yaka R, Gamliel A, Gurwitz D, Stein R . (1998). NGF induces transient but not sustained activation of ERK in PC12 mutant cells incapable of differentiating. J Cell Biochem 70: 425–432.
Yamada M, Numakawa T, Koshimizu H, Tanabe K, Wada K, Koizumi S et al. (2002). Distinct usages of phospholipase C gamma and Shc in intracellular signaling stimulated by neurotrophins. Brain Res 955: 183–190.
Acknowledgements
This work was supported by a Grant-in-Aid from the Ministry of Health, Labor and Welfare of Japan for the third-term Comprehensive 10-year Strategy for Cancer Control.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Miyake, I., Ohira, M., Nakagawara, A. et al. Distinct role of ShcC docking protein in the differentiation of neuroblastoma. Oncogene 28, 662–673 (2009). https://doi.org/10.1038/onc.2008.413
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.413