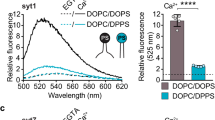
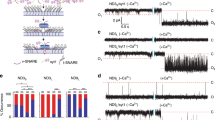
Abstract
Synaptotagmin-1 (syt), the putative Ca2+ sensor for exocytosis, is anchored to the membrane of secretory organelles. Its cytoplasmic domain is composed of two Ca2+-sensing modules, C2A and C2B. Syt binds phosphatidylinositol 4,5-bisphosphate (PIP2), a plasma membrane lipid with an essential role in exocytosis and endocytosis. We resolved two modes of PIP2 binding that are mediated by distinct surfaces on the C2B domain of syt. A novel Ca2+-independent mode of binding predisposes syt to penetrate PIP2-harboring target membranes in response to Ca2+ with submillisecond kinetics. Thus, PIP2 increases the speed of response of syt and steers its membrane-penetration activity toward the plasma membrane. We propose that syt-PIP2 interactions are involved in exocytosis by facilitating the close apposition of the vesicle and target membrane on rapid time scales in response to Ca2+.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Katz, B. The Release of Neural Transmitter Substances (Thomas, Springfield, Illinois, USA, 1969).
Augustine, G.J. How does calcium trigger neurotransmitter release? Curr. Opin. Neurobiol. 11, 320–326 (2001).
Malenka, R.C. & Nicoll, R.A. Silent synapses speak up. Neuron 19, 473–476 (1997).
Tong, G. & Jahr, C.E. Multivesicular release from excitatory synapses of cultured hippocampal neurons. Neuron 12, 51–59 (1994).
Choi, S., Klingauf, J. & Tsien, R.W. Postfusional regulation of cleft glutamate concentration during LTP at 'silent synapses'. Nat. Neurosci. 3, 330–336 (2000).
Renger, J.J., Egles, C. & Liu, G. A developmental switch in neurotransmitter flux enhances synaptic efficacy by affecting AMPA receptor activation. Neuron 29, 469–484 (2001).
Perin, M.S., Fried, V.A., Mignery, G.A., Jahn, R. & Sudhof, T.C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature 345, 260–263 (1990).
Craxton, M. Genomic analysis of synaptotagmin genes. Genomics 77, 43–49 (2001).
Brose, N., Petrenko, A.G., Sudhof, T.C. & Jahn, R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science 256, 1021–1025 (1992).
Chapman, E.R. Synaptotagmin: a Ca2+ sensor that triggers exocytosis? Nat. Rev. Mol. Cell Biol. 3, 498–508 (2002).
Littleton, J.T. et al. synaptotagmin mutants reveal essential functions for the C2B domain in Ca2+-triggered fusion and recycling of synaptic vesicles in vivo. J. Neurosci. 21, 1421–1433 (2001).
Fernandez-Chacon, R. et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41–49 (2001).
Mackler, J.M., Drummond, J.A., Loewen, C.A., Robinson, I.M. & Reist, N.E. The C(2)B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature 418, 340–344 (2002).
Robinson, I.M., Ranjan, R. & Schwarz, T.L. Synaptotagmins I and IV promote transmitter release independently of Ca2+ binding in the C(2)A domain. Nature 418, 336–340 (2002).
Fernandez-Chacon, R. et al. Structure/function analysis of Ca2+ binding to the C2A domain of synaptotagmin 1. J. Neurosci. 22, 8438–8446 (2002).
Yoshihara, M. & Littleton, J.T. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron 36, 897–908 (2002).
Wang, C.T. et al. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science 294, 1111–1115 (2001).
Schiavo, G., Gu, Q.M., Prestwich, G.D., Sollner, T.H. & Rothman, J.E. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc. Natl. Acad. Sci. USA 93, 13327–13332 (1996).
Holz, R.W. et al. A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J. Biol. Chem. 275, 17878–17885 (2000).
Micheva, K.D., Holz, R.W. & Smith, S.J. Regulation of presynaptic phosphatidylinositol 4,5-biphosphate by neuronal activity. J. Cell Biol. 154, 355–368 (2001).
Cremona, O. & De Camilli, P. Phosphoinositides in membrane traffic at the synapse. J. Cell Sci. 114, 1041–1052 (2001).
Eberhard, D.A., Cooper, C.L., Low, M.G. & Holz, R.W. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem. J. 268, 15–25 (1990).
Hay, J.C. & Martin, T.F. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca2+-activated secretion. Nature 366, 572–575 (1993).
Hay, J.C. et al. ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature 374, 173–177 (1995).
Zhang, X., Rizo, J. & Sudhof, T.C. Mechanism of phospholipid binding by the C2A-domain of synaptotagmin I. Biochemistry 37, 12395–12403 (1998).
McLaughlin, S., Wang, J., Gambhir, A. & Murray, D. PIP2 and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 31, 151–175 (2002).
Davis, A.F. et al. Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron 24, 363–376 (1999).
Bai, J., Wang, P. & Chapman, E.R. C2A activates a cryptic Ca2+-triggered membrane penetration activity within the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. USA 99, 1665–1670 (2002).
Bai, J., Earles, C.A., Lewis, J.L. & Chapman, E.R. Membrane-embedded synaptotagmin penetrates cis or trans target membranes and clusters via a novel mechanism. J. Biol. Chem. 275, 25427–25435 (2000).
Fernandez, I. et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain. Synaptotagmin 1 as a phospholipid binding machine. Neuron 32, 1057–1069 (2001).
Wu, Y. et al. Visualization of synaptotagmin I oligomers assembled onto lipid monolayers. Proc. Natl. Acad. Sci. USA 100, 2082–2087 (2003).
Fukuda, M., Kojima, T., Aruga, J., Niinobe, M. & Mikoshiba, K. Functional diversity of C2 domains of synaptotagmin family. Mutational analysis of inositol high polyphosphate binding domain. J. Biol. Chem. 270, 26523–26527 (1995).
Mackler, J.M. & Reist, N.E. Mutations in the second C2 domain of synaptotagmin disrupt synaptic transmission at Drosophila neuromuscular junctions. J. Comp. Neurol. 436, 4–16 (2001).
Desai, R.C. et al. The C2B domain of synaptotagmin is a Ca2+-sensing module essential for exocytosis. J. Cell Biol. 150, 1125–1136 (2000).
Tucker, W.C. et al. Identification of synaptotagmin effectors via acute inhibition of secretion from cracked PC12 cells. J. Cell Biol. 162, 199–209 (2003).
Fukuda, M. et al. Role of the C2B domain of synaptotagmin in vesicular release and recycling as determined by specific antibody injection into the squid giant synapse preterminal. Proc. Natl. Acad. Sci. USA 92, 10708–10712 (1995).
Bommert, K. et al. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature 363, 163–165 (1993).
Loyet, K.M. et al. Specific binding of phosphatidylinositol 4,5-bisphosphate to calcium-dependent activator protein for secretion (CAPS), a potential phosphoinositide effector protein for regulated exocytosis. J. Biol. Chem. 273, 8337–8343 (1998).
Chung, S.H. et al. The C2 domains of Rabphilin3A specifically bind phosphatidylinositol 4,5-bisphosphate containing vesicles in a Ca2+-dependent manner. In vitro characteristics and possible significance. J. Biol. Chem. 273, 10240–10248 (1998).
Burns, M.E., Sasaki, T., Takai, Y. & Augustine, G.J. Rabphilin-3A: a multifunctional regulator of synaptic vesicle traffic. J. Gen. Physiol. 111, 243–255 (1998).
Schluter, O.M. et al. Rabphilin knock-out mice reveal that rabphilin is not required for rab3 function in regulating neurotransmitter release. J. Neurosci. 19, 5834–5846 (1999).
Chapman, E.R. & Jahn, R. Calcium-dependent interaction of the cytoplasmic region of synaptotagmin with membranes. Autonomous function of a single C2-homologous domain. J. Biol. Chem. 269, 5735–5741 (1994).
Wang, P., Wang, C.T., Bai, J., Jackson, M.B. & Chapman, E.R. Mutations in the effector binding loops in the C2A and C2B domains of synaptotagmin I disrupt exocytosis in a non-additive manner. J. Biol. Chem. (2003).
Mahal, L.K., Sequeira, S.M., Gureasko, J.M. & Sollner, T.H. Calcium-independent stimulation of membrane fusion and SNAREpin formation by synaptotagmin I. J. Cell Biol. 158, 273–282 (2002).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Chen, Y.A., Scales, S.J., Patel, S.M., Doung, Y.C. & Scheller, R.H. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell 97, 165–174 (1999).
Earles, C.A., Bai, J., Wang, P. & Chapman, E.R. The tandem C2 domains of synaptotagmin contain redundant Ca2+ binding sites that cooperate to engage t-SNAREs and trigger exocytosis. J. Cell Biol. 154, 1117–1123 (2001).
Zhang, X., Kim-Miller, M.J., Fukuda, M., Kowalchyk, J.A. & Martin, T.F. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron 34, 599–611 (2002).
Chapman, E.R. & Davis, A.F. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 273, 13995–14001 (1998).
Wiedemann, C., Schafer, T., Burger, M.M. & Sihra, T.S. An essential role for a small synaptic vesicle-associated phosphatidylinositol 4-kinase in neurotransmitter release. J. Neurosci. 18, 5594–5602 (1998).
Khvotchev, M. & Sudhof, T.C. Newly synthesized phosphatidylinositol phosphates are required for synaptic norepinephrine but not glutamate or γ-aminobutyric acid (GABA) release. J. Biol. Chem. 273, 21451–21454 (1998).
Elferink, L.A., Trimble, W.S. & Scheller, R.H. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J. Biol. Chem. 264, 11061–11064 (1989).
Chapman, E.R., Hanson, P.I., An, S. & Jahn, R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J. Biol. Chem. 270, 23667–23671 (1995).
Ubach, J. et al. The C2B domain of synaptotagmin I is a Ca2+-binding module. Biochemistry 40, 5854–5860 (2001).
Shao, X., Fernandez, I., Sudhof, T.C. & Rizo, J. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change? Biochemistry 37, 16106–16115 (1998).
Acknowledgements
We thank J.M. Edwardson, T.F.J. Martin, M. Jackson and C.T. Wang for helpful discussions, and R. Jahn and S. Engers for synaptobrevin-2 antibodies. This study was supported by grants from the US National Institutes of Health (National Institute of General Medical Sciences GM 56827 and National Institute of Mental Health MH61876), American Heart Association (AHA) 9750326N and the Milwaukee Foundation. E.R.C. is a Pew Scholar in the Biomedical Sciences. J.B. is supported by an AHA predoctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Bai, J., Tucker, W. & Chapman, E. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat Struct Mol Biol 11, 36–44 (2004). https://doi.org/10.1038/nsmb709
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb709
This article is cited by
-
Mechanisms of SNARE proteins in membrane fusion
Nature Reviews Molecular Cell Biology (2024)
-
The release of inhibition model reproduces kinetics and plasticity of neurotransmitter release in central synapses
Communications Biology (2023)
-
Synaptotagmin-7 outperforms synaptotagmin-1 to promote the formation of large, stable fusion pores via robust membrane penetration
Nature Communications (2023)
-
Vesicle trafficking and vesicle fusion: mechanisms, biological functions, and their implications for potential disease therapy
Molecular Biomedicine (2022)
-
The complexin C-terminal amphipathic helix stabilizes the fusion pore open state by sculpting membranes
Nature Structural & Molecular Biology (2022)