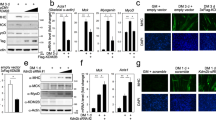
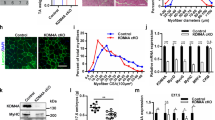
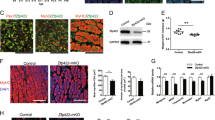
Abstract
Cell-specific patterns of gene expression are established through the antagonistic functions of trithorax group (TrxG) and Polycomb group (PcG) proteins. Several muscle-specific genes have previously been shown to be epigenetically marked for repression by PcG proteins in muscle progenitor cells. Here we demonstrate that these developmentally regulated genes become epigenetically marked for gene expression (trimethylated on histone H3 Lys4, H3K4me3) during muscle differentiation through specific recruitment of Ash2L-containing methyltransferase complexes. Targeting of Ash2L to specific genes is mediated by the transcriptional regulator Mef2d. Furthermore, this interaction is modulated during differentiation through activation of the p38 MAPK signaling pathway via phosphorylation of Mef2d. Thus, we provide evidence that signaling pathways regulate the targeting of TrxG-mediated epigenetic modifications at specific promoters during cellular differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ringrose, L. & Paro, R. Epigenetic regulation of cellular memory by the polycomb and trithorax group proteins. Annu. Rev. Genet. 38, 413–443 (2004).
Brock, H.W. & Fisher, C.L. Maintenance of gene expression patterns. Dev. Dyn. 232, 633–655 (2005).
Ruthenburg, A.J., Allis, C.D. & Wysocka, J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol. Cell 25, 15–30 (2007).
Bernstein, B.E. et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120, 169–181 (2005).
Martens, J.H. et al. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J. 24, 800–812 (2005).
Papp, B. & Muller, J. Histone trimethylation and the maintenance of transcriptional ON and OFF states by trxG and PcG proteins. Genes Dev. 20, 2041–2054 (2006).
Breen, T.R. & Harte, P.J. Molecular characterization of the trithorax gene, a positive regulator of homeotic gene expression in Drosophila. Mech. Dev. 35, 113–127 (1991).
Klymenko, T. & Muller, J. The histone methyltransferases Trithorax and Ash1 prevent transcriptional silencing by Polycomb group proteins. EMBO Rep. 5, 373–377 (2004).
Molkentin, J.D., Black, B.L., Martin, J.F. & Olson, E.N. Cooperative activation of muscle gene expression by MEf2 and myogenic bHLH proteins. Cell 83, 1125–1136 (1995).
Bergstrom, D.A. et al. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell 9, 587–600 (2002).
Penn, B.H., Bergstrom, D.A., Dilworth, F.J., Bengal, E. & Tapscott, S.J.A. MyoD-generated feed forward circuit temporally patterns gene expression during skeletal muscle differentiation. Genes Dev. 18, 2348–2353 (2004).
Wu, Z. et al. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 20, 3951–3964 (2000).
Zetser, A., Gredinger, E. & Bengal, E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 274, 5193–5200 (1999).
Caretti, G., Di Padova, M., Micales, B., Lyons, G.E. & Sartorelli, V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 18, 2627–2638 (2004).
Zhang, C.L., McKinsey, T.A. & Olson, E.N. Association of class II histone deacetylases with heterochromatin protein 1: potential role for histone methylation in control of muscle differentiation. Mol. Cell. Biol. 22, 7302–7312 (2002).
Guenther, M.G., Levine, S.S., Boyer, L.A., Jaenisch, R. & Young, R.A. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130, 77–88 (2007).
Yaffe, D. & Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725–727 (1977).
Milne, T.A. et al. MLL associates specifically with a subset of transcriptionally active target genes. Proc. Natl. Acad. Sci. USA 102, 14765–14770 (2005).
Steward, M.M. et al. Molecular regulation of H3K4 trimethylation by ASH2L, a shared subunit of MLL complexes. Nat. Struct. Mol. Biol. 13, 852–854 (2006).
Dou, Y. et al. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat. Struct. Mol. Biol. 13, 713–719 (2006).
Wang, J. et al. ASH2L: alternative splicing and downregulation during induced megakaryocytic differentiation of multipotential leukemia cell lines. J. Mol. Med. 79, 399–405 (2001).
Cheng, T.C., Wallace, M.C., Merlie, J.P. & Olson, E.N. Separable regulatory elements governing myogenin transcription in mouse embryogenesis. Science 261, 215–218 (1993).
Yee, S.P. & Rigby, P.W. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 7, 1277–1289 (1993).
Edmondson, D.G., Cheng, T.C., Cserjesi, P., Chakraborty, T. & Olson, E.N. Analysis of the myogenin promoter reveals an indirect pathway for positive autoregulation mediated by the muscle-specific enhancer factor MEF-2. Mol. Cell. Biol. 12, 3665–3677 (1992).
Malik, S., Huang, C.F. & Schmidt, J. The role of the CANNTG promoter element (E box) and the myocyte-enhancer-binding-factor-2 (MEF-2) site in the transcriptional regulation of the chick myogenin gene. Eur. J. Biochem. 230, 88–96 (1995).
Berkes, C.A. et al. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol. Cell 14, 465–477 (2004).
Cho, Y.W. et al. PTIP associates with MLL3- and MLL4-containing histone H3 lysine 4 methyltransferase complex. J. Biol. Chem. 282, 20395–20406 (2007).
Zhao, M. et al. Regulation of the MEf2 family of transcription factors by p38. Mol. Cell. Biol. 19, 21–30 (1999).
Khokhlatchev, A. et al. Reconstitution of mitogen-activated protein kinase phosphorylation cascades in bacteria. Efficient synthesis of active protein kinases. J. Biol. Chem. 272, 11057–11062 (1997).
Zhu, B., Ramachandran, B. & Gulick, T. Alternative pre-mRNA splicing governs expression of a conserved acidic transactivation domain in myocyte enhancer factor 2 factors of striated muscle and brain. J. Biol. Chem. 280, 28749–28760 (2005).
Simone, C. et al. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat. Genet. 36, 738–743 (2004).
Ernst, P., Wang, J., Huang, M., Goodman, R.H. & Korsmeyer, S.J. MLL and CREB bind cooperatively to the nuclear coactivator CREB-binding protein. Mol. Cell. Biol. 21, 2249–2258 (2001).
Voo, K.S., Carlone, D.L., Jacobsen, B.M., Flodin, A. & Skalnik, D.G. Cloning of a mammalian transcriptional activator that binds unmethylated CpG motifs and shares a CXXC domain with DNA methyltransferase, human trithorax, and methyl-CpG binding domain protein 1. Mol. Cell. Biol. 20, 2108–2121 (2000).
Wysocka, J. et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell 121, 859–872 (2005).
Lluis, F., Perdiguero, E. & Nebreda, A.R. Munoz-Canoves, P. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 16, 36–44 (2006).
Takaesu, G. et al. Activation of p38α/β MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J. Cell Biol. 175, 383–388 (2006).
Ohkawa, Y., Marfella, C.G. & Imbalzano, A.N. Skeletal muscle specification by myogenin and Mef2D via the SWI/SNF ATPase Brg1. EMBO J. 25, 490–501 (2006).
de la Serna, I.L. et al. MyoD targets chromatin remodeling complexes to the myogenin locus prior to forming a stable DNA-bound complex. Mol. Cell. Biol. 25, 3997–4009 (2005).
Brand, M. et al. Dynamic changes in transcription factor complexes during erythroid differentiation revealed by quantitative proteomics. Nat. Struct. Mol. Biol. 11, 73–80 (2004).
Dilworth, F.J., Ramain-Fromental, C., Yamamoto, K. & Chambon, P. ATP-driven chromatin remodeling activity and histone acetyltransferases act sequencially during transactivation by RAR/RXR in vitro. Mol. Cell 6, 1049–1058 (2000).
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research, the Muscular Dystrophy Association and the Stem Cell Network (to F.J.D.), from the US National Institutes of Health (to S.J.T.) and from the Terry Fox Foundation of the National Cancer Institute of Canada and the Human Frontiers Science Program (to M.B.). F.J.D. holds a Canadian Research Chair in Epigenetic Regulation of Transcription.
Author information
Authors and Affiliations
Contributions
S.R. and F.J.D. conceived and designed the experiments. S.R. was responsible for all ChIP, immunoprecipitation, RT-PCR and interaction studies. F.J.D. and M.B. generated the polyclonal antibody to full-length Ash2L. L.L. generated the polyclonal antibodies to full-length DPY and MyoD. E.M. generated the Mef2D mutant. S.R. and E.M. performed the transfection studies with the Mef2 mutants. K.G. provided antibodies to MLL3 and MLL4. S.R., M.B., S.J.T. and F.J.D. provided scientific direction of the project. F.J.D. wrote the paper. S.R., M.B. and F.J.D. discussed and commented on the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Table 1 (PDF 518 kb)
Rights and permissions
About this article
Cite this article
Rampalli, S., Li, L., Mak, E. et al. p38 MAPK signaling regulates recruitment of Ash2L-containing methyltransferase complexes to specific genes during differentiation. Nat Struct Mol Biol 14, 1150–1156 (2007). https://doi.org/10.1038/nsmb1316
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1316
This article is cited by
-
lncCPSET1 acts as a scaffold for MLL2/COMPASS to regulate Bmp4 and promote the formation of chicken primordial germ cells
Molecular Genetics and Genomics (2024)
-
Epigenetic modifications in muscle regeneration and progression of Duchenne muscular dystrophy
Clinical Epigenetics (2021)
-
Epigenetic regulation of satellite cell fate during skeletal muscle regeneration
Skeletal Muscle (2021)
-
Smad7:β-catenin complex regulates myogenic gene transcription
Cell Death & Disease (2019)
-
Diversification of the muscle proteome through alternative splicing
Skeletal Muscle (2018)