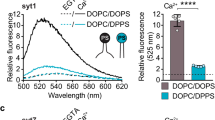
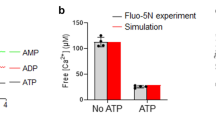
Abstract
Synaptotagmin-1 is the calcium sensor for neuronal exocytosis, but the mechanism by which it triggers membrane fusion is not fully understood. Here we show that synaptotagmin accelerates SNARE-dependent fusion of liposomes by interacting with neuronal Q-SNARES in a Ca2+-independent manner. Ca2+-dependent binding of synaptotagmin to its own membrane impedes the activation. Preventing this cis interaction allows Ca2+ to trigger synaptotagmin binding in trans, accelerating fusion. However, when an activated SNARE acceptor complex is used, synaptotagmin has no effect on fusion kinetics, suggesting that synaptotagmin operates upstream of SNARE assembly in this system. Our results resolve major discrepancies concerning the effects of full-length synaptotagmin and its C2AB fragment on liposome fusion and shed new light on the interactions of synaptotagmin with SNAREs and membranes. However, our findings also show that the action of synaptotagmin on the fusion-arrested state of docked vesicles in vivo is not fully reproduced in vitro.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Ungar, D. & Hughson, F.M. SNARE protein structure and function. Annu. Rev. Cell Dev. Biol. 19, 493–517 (2003).
Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547 (2004).
Fasshauer, D., Otto, H., Eliason, W.K., Jahn, R. & Brunger, A.T. Structural changes are associated with soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor complex formation. J. Biol. Chem. 272, 28036–28041 (1997).
Sutton, R.B., Fasshauer, D., Jahn, R. & Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353 (1998).
Fasshauer, D., Sutton, R.B., Brunger, A.T. & Jahn, R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. USA 95, 15781–15786 (1998).
Pelham, H.R., Banfield, D.K. & Lewis, M.J. SNAREs involved in traffic through the Golgi complex. Cold Spring Harb. Symp. Quant. Biol. 60, 105–111 (1995).
Hanson, P.I., Heuser, J.E. & Jahn, R. Neurotransmitter release—four years of SNARE complexes. Curr. Opin. Neurobiol. 7, 310–315 (1997).
Lin, R.C. & Scheller, R.H. Structural organization of the synaptic exocytosis core complex. Neuron 19, 1087–1094 (1997).
Katz, B. The Release of Neurotransmitter Substances (Liverpool University Press, Liverpool, 1969).
Sudhof, T.C. Synaptotagmins: why so many? J. Biol. Chem. 277, 7629–7632 (2002).
Perin, M.S., Brose, N., Jahn, R. & Sudhof, T.C. Domain structure of synaptotagmin (p65). J. Biol. Chem. 266, 623–629 (1991).
Tucker, W.C. & Chapman, E.R. Role of synaptotagmin in Ca2+-triggered exocytosis. Biochem. J. 366, 1–13 (2002).
Ubach, J., Zhang, X., Shao, X., Sudhof, T.C. & Rizo, J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 17, 3921–3930 (1998).
Fernandez, I. et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron 32, 1057–1069 (2001).
Chapman, E.R. & Davis, A.F. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 273, 13995–14001 (1998).
Rufener, E., Frazier, A.A., Wieser, C.M., Hinderliter, A. & Cafiso, D.S. Membrane-bound orientation and position of the synaptotagmin C2B domain determined by site-directed spin labeling. Biochemistry 44, 18–28 (2005).
Herrick, D.Z., Sterbling, S., Rasch, K.A., Hinderliter, A. & Cafiso, D.S. Position of synaptotagmin I at the membrane interface: cooperative interactions of tandem C2 domains. Biochemistry 45, 9668–9674 (2006).
Li, L. et al. Phosphatidylinositol phosphates as co-activators of Ca2+ binding to C2 domains of synaptotagmin 1. J. Biol. Chem. 281, 15845–15852 (2006).
Pang, Z.P., Shin, O.H., Meyer, A.C., Rosenmund, C. & Sudhof, T.C. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J. Neurosci. 26, 12556–12565 (2006).
Bai, J., Tucker, W.C. & Chapman, E.R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36–44 (2004).
Chapman, E.R., Hanson, P.I., An, S. & Jahn, R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J. Biol. Chem. 270, 23667–23671 (1995).
Schiavo, G., Stenbeck, G., Rothman, J.E. & Sollner, T.H. Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses. Proc. Natl. Acad. Sci. USA 94, 997–1001 (1997).
Rickman, C. & Davletov, B. Mechanism of calcium-independent synaptotagmin binding to target SNAREs. J. Biol. Chem. 278, 5501–5504 (2003).
Davis, A.F. et al. Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron 24, 363–376 (1999).
Stevens, C.F. & Sullivan, J.M. The synaptotagmin C2A domain is part of the calcium sensor controlling fast synaptic transmission. Neuron 39, 299–308 (2003).
Mackler, J.M., Drummond, J.A., Loewen, C.A., Robinson, I.M. & Reist, N.E. The C(2)B Ca(2+)-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature 418, 340–344 (2002).
Fernandez-Chacon, R. et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41–49 (2001).
Rhee, J.S. et al. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proc. Natl. Acad. Sci. USA 102, 18664–18669 (2005).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Schuette, C.G. et al. Determinants of liposome fusion mediated by synaptic SNARE proteins. Proc. Natl. Acad. Sci. USA 101, 2858–2863 (2004).
Jahn, R. & Scheller, R.H. SNAREs–engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7, 631–643 (2006).
Tucker, W.C., Weber, T. & Chapman, E.R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435–438 (2004).
Bhalla, A., Tucker, W.C. & Chapman, E.R. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Mol. Biol. Cell 16, 4755–4764 (2005).
Bhalla, A., Chicka, M.C., Tucker, W.C. & Chapman, E.R. Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 13, 323–330 (2006).
Mahal, L.K., Sequeira, S.M., Gureasko, J.M. & Sollner, T.H. Calcium-independent stimulation of membrane fusion and SNAREpin formation by synaptotagmin I. J. Cell Biol. 158, 273–282 (2002).
Struck, D.K., Hoekstra, D. & Pagano, R.E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry 20, 4093–4099 (1981).
Hayashi, T. et al. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 13, 5051–5061 (1994).
Antonin, W., Holroyd, C., Tikkanen, R., Honing, S. & Jahn, R. The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes. Mol. Biol. Cell 11, 3289–3298 (2000).
Pobbati, A.V., Stein, A. & Fasshauer, D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science 313, 673–676 (2006).
Rickman, C. et al. Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 279, 12574–12579 (2004).
Loewen, C.A., Lee, S.M., Shin, Y.K. & Reist, N.E. C2B polylysine motif of synaptotagmin facilitates a Ca2+-independent stage of synaptic vesicle priming in vivo. Mol. Biol. Cell 17, 5211–5226 (2006).
Yoon, T.Y., Okumus, B., Zhang, F., Shin, Y.K. & Ha, T. Multiple intermediates in SNARE-induced membrane fusion. Proc. Natl. Acad. Sci. USA 103, 19731–19736 (2006).
Arac, D. et al. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 13, 209–217 (2006).
Martens, S., Kozlov, M.M. & McMahon, H.T. How synaptotagmin promotes membrane fusion. Science 316, 1205–1208 (2007).
Margittai, M., Otto, H. & Jahn, R. A stable interaction between syntaxin 1a and synaptobrevin 2 mediated by their transmembrane domains. FEBS Lett. 446, 40–44 (1999).
Fasshauer, D., Antonin, W., Margittai, M., Pabst, S. & Jahn, R. Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J. Biol. Chem. 274, 15440–15446 (1999).
Brandhorst, D. et al. Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity. Proc. Natl. Acad. Sci. USA 103, 2701–2706 (2006).
Sieber, J.J., Willig, K.I., Heintzmann, R., Hell, S.W. & Lang, T. The SNARE motif is essential for the formation of syntaxin clusters in the plasma membrane. Biophys. J. 90, 2843–2851 (2006).
Takamori, S. et al. Molecular anatomy of a trafficking organelle. Cell 127, 831–846 (2006).
Schagger, H. & von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368–379 (1987).
Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Avery, J. et al. A cell-free system for regulated exocytosis in PC12 cells. J. Cell Biol. 148, 317–324 (2000).
Acknowledgements
We thank U. Ries for expert technical assistance, M. Holt for help with calcium measurements, S. Pabst for cloning, and P. Burkhardt and I. Bethani for valuable comments on the manuscript. Ca2+-binding mutants of the full-length protein were kind gifts from T. Südhof (Howard Hughes Medical Institute, University of Texas Southwestern). This work was supported by US National Institutes of Health grant P01 GM072694 (to R.J.) and the Boehringer Ingelheim Fonds (to A.S.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Methods (PDF 11704 kb)
Rights and permissions
About this article
Cite this article
Stein, A., Radhakrishnan, A., Riedel, D. et al. Synaptotagmin activates membrane fusion through a Ca2+-dependent trans interaction with phospholipids. Nat Struct Mol Biol 14, 904–911 (2007). https://doi.org/10.1038/nsmb1305
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1305
This article is cited by
-
Electrostatic regulation of the cis- and trans-membrane interactions of synaptotagmin-1
Scientific Reports (2022)
-
Regulation of the mammalian-brain V-ATPase through ultraslow mode-switching
Nature (2022)
-
Cross-linking mass spectrometry uncovers protein interactions and functional assemblies in synaptic vesicle membranes
Nature Communications (2021)
-
Probing the effect of a room temperature ionic liquid on phospholipid membranes in multilamellar vesicles
European Biophysics Journal (2019)
-
A molecular mechanism for calcium-mediated synaptotagmin-triggered exocytosis
Nature Structural & Molecular Biology (2018)