Abstract
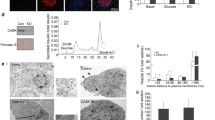
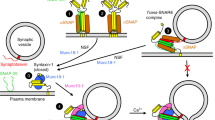
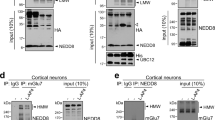
Munc13 proteins are essential in neurotransmitter release, controlling the priming of synaptic vesicles to a release-ready state. The sequences responsible for this priming activity are unknown. Here we identify a large α-helical domain of mammalian Munc13-1 that is autonomously folded and is sufficient to rescue the total arrest in neurotransmitter release observed in hippocampal neurons lacking Munc13s.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rizo, J. & Sudhof, T.C. Nat. Rev. Neurosci. 3, 641–653 (2002).
Dulubova, I. et al. EMBO J. 18, 4372–4382 (1999).
Varoqueaux, F. et al. Proc. Natl. Acad. Sci. USA 99, 9037–9042 (2002).
Rosenmund, C. et al. Neuron 33, 411–424 (2002).
Rhee, J.S. et al. Cell 108, 121–133 (2002).
Junge, H.J. et al. Cell 118, 389–401 (2004).
Koch, H., Hofmann, K. & Brose, N. Biochem. J. 349, 247–253 (2000).
Richmond, J.E., Weimer, R.M. & Jorgensen, E.M. Nature 412, 338–341 (2001).
Betz, A., Okamoto, M., Benseler, F. & Brose, N. J. Biol. Chem. 272, 2520–2526 (1997).
Betz, A. et al. Neuron 30, 183–196 (2001).
Dulubova, I. et al. EMBO J. 24, 2839–2850 (2005).
Feldmann, J. et al. Cell 115, 461–473 (2003).
Speidel, D. et al. Neuron 46, 75–88 (2005).
Acknowledgements
We thank T. Südhof for fruitful discussions, H. Deng and R. Nehring for technical support and N. Brose (Max Planck Institute for Experimental Medicine, Gottingen, Germany) for providing the Munc13-1 and Munc13-2 double-knockout mice and the pSFV1-Munc13-1WT-EGFP vector. This work was supported by a grant from the Muscular Dystrophy Association and by US National Institutes of Health grants NS37200 to J.R. and NS51262 to C.R.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Munc13-1 fragments that did not fold (PDF 38 kb)
Supplementary Fig. 2
Secondary structure prediction of Munc13-1 (800-1579) (PDF 38 kb)
Supplementary Fig. 3
Purification of the MUN domain (PDF 18 kb)
Supplementary Fig. 4
The MUN domain does not bind syntaxin (PDF 137 kb)
Supplementary Methods
Computational analysis, cell culture and electrophysiology (PDF 28 kb)
Rights and permissions
About this article
Cite this article
Basu, J., Shen, N., Dulubova, I. et al. A minimal domain responsible for Munc13 activity. Nat Struct Mol Biol 12, 1017–1018 (2005). https://doi.org/10.1038/nsmb1001
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1001
This article is cited by
-
Vesicle trafficking and vesicle fusion: mechanisms, biological functions, and their implications for potential disease therapy
Molecular Biomedicine (2022)
-
Inhibition of calcium-triggered secretion by hydrocarbon-stapled peptides
Nature (2022)
-
Autaptic cultures of human induced neurons as a versatile platform for studying synaptic function and neuronal morphology
Scientific Reports (2019)
-
The presynaptic machinery at the synapse of C. elegans
Invertebrate Neuroscience (2018)
-
Vertebrate Presynaptic Active Zone Assembly: a Role Accomplished by Diverse Molecular and Cellular Mechanisms
Molecular Neurobiology (2018)