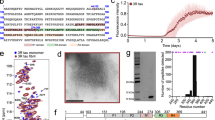
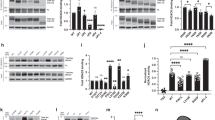
Abstract
Tau proteins are the building blocks of neurofibrillary tangles (NFTs) found in a range of neurodegenerative tauopathies, including Alzheimer's disease. Recently, we demonstrated that tau is extensively post-translationally modified by lysine acetylation, which impairs normal tau function and promotes pathological aggregation. Identifying the enzymes that mediate tau acetylation could provide targets for future therapies aimed at reducing the burden of acetylated tau. Here, we report that mammalian tau proteins possess intrinsic enzymatic activity capable of catalyzing self-acetylation. Functional mapping of tau acetyltransferase activity followed by biochemical analysis revealed that tau uses catalytic cysteine residues in the microtubule-binding domain to facilitate tau lysine acetylation, thus suggesting a mechanism similar to that employed by MYST-family acetyltransferases. The identification of tau as an acetyltransferase provides a framework to further understand tau pathogenesis and highlights tau enzymatic activity as a potential therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Andreadis, A., Brown, W.M. & Kosik, K.S. Structure and novel exons of the human tau gene. Biochemistry 31, 10626–10633 (1992).
Goedert, M., Spillantini, M.G., Jakes, R., Rutherford, D. & Crowther, R.A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3, 519–526 (1989).
Lee, V.M. Regulation of tau phosphorylation in Alzheimer's disease. Ann. NY Acad. Sci. 777, 107–113 (1996).
Lee, V.M., Goedert, M. & Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159 (2001).
Cohen, T.J. et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2, 252 (2011).
Irwin, D.J. et al. Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies. Brain 135, 807–818 (2012).
Min, S.W. et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966 (2010).
Brownell, J.E. & Allis, C.D. An activity gel assay detects a single, catalytically active histone acetyltransferase subunit in Tetrahymena macronuclei. Proc. Natl. Acad. Sci. USA 92, 6364–6368 (1995).
Brownell, J.E., Mizzen, C.A. & Allis, C.D. An SDS-PAGE-based enzyme activity assay for the detection and identification of histone acetyltransferases. Methods Mol. Biol. 119, 343–353 (1999).
Kawahara, T. et al. Two essential MYST-family proteins display distinct roles in histone H4K10 acetylation and telomeric silencing in trypanosomes. Mol. Microbiol. 69, 1054–1068 (2008).
Sterner, D.E. & Berger, S.L. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64, 435–459 (2000).
Sternglanz, R. & Schindelin, H. Structure and mechanism of action of the histone acetyltransferase Gcn5 and similarity to other N-acetyltransferases. Proc. Natl. Acad. Sci. USA 96, 8807–8808 (1999).
Yan, Y., Barlev, N.A., Haley, R.H., Berger, S.L. & Marmorstein, R. Crystal structure of yeast Esa1 suggests a unified mechanism for catalysis and substrate binding by histone acetyltransferases. Mol. Cell 6, 1195–1205 (2000).
Yan, Y., Harper, S., Speicher, D.W. & Marmorstein, R. The catalytic mechanism of the ESA1 histone acetyltransferase involves a self-acetylated intermediate. Nat. Struct. Biol. 9, 862–869 (2002).
Decker, P.V., Yu, D.Y., Iizuka, M., Qiu, Q. & Smith, M.M. Catalytic-site mutations in the MYST family histone acetyltransferase Esa1. Genetics 178, 1209–1220 (2008).
Yang, C., Wu, J. & Zheng, Y.G. Function of the active site lysine autoacetylation in tip60 catalysis. PLoS ONE 7, e32886 (2012).
Wang, Y. et al. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum. Mol. Genet. 18, 4153–4170 (2009).
Giasson, B.I. et al. The environmental toxin arsenite induces tau hyperphosphorylation. Biochemistry 41, 15376–15387 (2002).
Shida, T., Cueva, J.G., Xu, Z., Goodman, M.B. & Nachury, M.V. The major α-tubulin K40 acetyltransferase αTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. USA 107, 21517–21522 (2010).
Drewes, G., Ebneth, A., Preuss, U., Mandelkow, E.M. & Mandelkow, E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89, 297–308 (1997).
Akella, J.S. et al. MEC-17 is an α-tubulin acetyltransferase. Nature 467, 218–222 (2010).
Choudhary, C. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 (2009).
Congdon, E.E. et al. Nucleation-dependent tau filament formation: the importance of dimerization and an estimation of elementary rate constants. J. Biol. Chem. 283, 13806–13816 (2008).
Corrigan, F.M., Horrobin, D.F., Skinner, E.R., Besson, J.A. & Cooper, M.B. Abnormal content of n-6 and n-3 long-chain unsaturated fatty acids in the phosphoglycerides and cholesterol esters of parahippocampal cortex from Alzheimer's disease patients and its relationship to acetyl CoA content. Int. J. Biochem. Cell Biol. 30, 197–207 (1998).
Kawasaki, H. et al. ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature 405, 195–200 (2000).
Lemercier, C. et al. Tip60 acetyltransferase activity is controlled by phosphorylation. J. Biol. Chem. 278, 4713–4718 (2003).
Drewes, G. et al. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J. Biol. Chem. 270, 7679–7688 (1995).
Mandelkow, E.M. et al. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol. Aging 16, 355–362, discussion 362–363 (1995).
Fischer, D. et al. Conformational changes specific for pseudophosphorylation at serine 262 selectively impair binding of tau to microtubules. Biochemistry 48, 10047–10055 (2009).
Mandelkow, E.M. & Mandelkow, E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2, a006247 (2012).
Schneider, A., Biernat, J., von Bergen, M., Mandelkow, E. & Mandelkow, E.M. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38, 3549–3558 (1999).
Cohen, T.J., Hwang, A.W., Unger, T., Trojanowski, J.Q. & Lee, V.M. Redox signalling directly regulates TDP-43 via cysteine oxidation and disulphide cross-linking. EMBO J. 31, 1241–1252 (2012).
Acknowledgements
We would like to thank T.P. Yao, J.Q. Trojanowski and L.K. Kwong for critical reading of this manuscript. We thank T.P. Yao (Duke University, Durham, North Carolina, USA) for kindly providing plasmids expressing a panel of histone acetyltransferases. We thank P. Seubert (Elan Pharmaceuticals, San Francisco, California, USA) for kindly providing anti-tau 12e8 antibody. We thank K. Brunden, A. Crowe, C. Li and other members of the Center for Neurodegenerative Disease Research for their technical support, helpful comments and critical suggestions. This study was supported by the US National Institutes of Health grants AG17586 (V.M.Y.L.) and GM060293 (R.M.) and the Association for Frontotemporal Degeneration (T.J.C.).
Author information
Authors and Affiliations
Contributions
T.J.C. and A.W.H. performed in vitro and cell-based acetylation experiments, mouse and human biochemical procedures and MS analysis, and T.J.C. was involved in the design and writing of this study. D.F. performed kinetic analysis of tau acetyltransferase activity and was involved in the writing of this study. V.M.Y.L. and R.M. supervised and designed the experiments and were involved in the writing of this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Table 1 (PDF 6124 kb)
Rights and permissions
About this article
Cite this article
Cohen, T., Friedmann, D., Hwang, A. et al. The microtubule-associated tau protein has intrinsic acetyltransferase activity. Nat Struct Mol Biol 20, 756–762 (2013). https://doi.org/10.1038/nsmb.2555
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2555
This article is cited by
-
Acetylation discriminates disease-specific tau deposition
Nature Communications (2023)
-
Mical modulates Tau toxicity via cysteine oxidation in vivo
Acta Neuropathologica Communications (2022)
-
The Role of Post-Translational Modifications on the Structure and Function of Tau Protein
Journal of Molecular Neuroscience (2022)
-
Tau K321/K353 pseudoacetylation within KXGS motifs regulates tau–microtubule interactions and inhibits aggregation
Scientific Reports (2021)
-
Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease
Molecular Neurodegeneration (2020)