Abstract
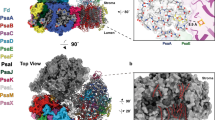
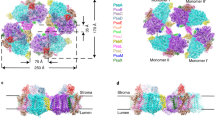
Photosystem II (PSII) is a large homodimeric protein–cofactor complex located in the photosynthetic thylakoid membrane that acts as light-driven water:plastoquinone oxidoreductase. The crystal structure of PSII from Thermosynechococcus elongatus at 2.9-Å resolution allowed the unambiguous assignment of all 20 protein subunits and complete modeling of all 35 chlorophyll a molecules and 12 carotenoid molecules, 25 integral lipids and 1 chloride ion per monomer. The presence of a third plastoquinone QC and a second plastoquinone-transfer channel, which were not observed before, suggests mechanisms for plastoquinol-plastoquinone exchange, and we calculated other possible water or dioxygen and proton channels. Putative oxygen positions obtained from a Xenon derivative indicate a role for lipids in oxygen diffusion to the cytoplasmic side of PSII. The chloride position suggests a role in proton-transfer reactions because it is bound through a putative water molecule to the Mn4Ca cluster at a distance of 6.5 Å and is close to two possible proton channels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wydrzynski, T. & Satoh, K. (eds.) Photosystem II: The Light-Driven Water:Plastoquinone Oxidoreductase (Springer, Dordrecht, 2005).
Kern, J. & Renger, G. Photosystem II. Structure and mechanism of the water:plastoquinone oxidoreductase. Photosynth. Res. 94, 183–202 (2007).
Renger, G. Oxidative photosynthetic water splitting: energetics, kinetics and mechanism. Photosynth. Res. 92, 407–425 (2007).
Brudvig, G.W. Water oxidation chemistry of photosystem II. Phil. Trans. R. Soc. Lond. B 363, 1211–1218 (2008).
Zouni, A. et al. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature 409, 739–743 (2001).
Kamiya, N. & Shen, J.R. Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7 Å resolution. Proc. Natl. Acad. Sci. USA 100, 98–103 (2003).
Ferreira, K.N., Iverson, T.M., Maghlaoui, K., Barber, J. & Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 (2004).
Biesiadka, J., Loll, B., Kern, J., Irrgang, K.-D. & Zouni, A. Crystal structure of cyanobacterial photosystem II at 3.2 Å resolution: a closer look at the Mn-cluster. Phys. Chem. Chem. Phys. 6, 4733–4736 (2004).
Loll, B., Kern, J., Saenger, W., Zouni, A. & Biesiadka, J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 438, 1040–1044 (2005).
Kashino, Y. et al. Ycf12 is a core subunit in the photosystem II complex. Biochim. Biophys. Acta 1767, 1269–1275 (2007).
Loll, B., Kern, J., Saenger, W., Zouni, A. & Biesiadka, J. Lipids in photosystem II: interactions with protein and cofactors. Biochim. Biophys. Acta 1767, 509–519 (2007).
Sakurai, I. et al. Lipids in oxygen-evolving photosystem II complexes of cyanobacteria and higher plants. J. Biochem. 140, 201–209 (2006).
Gurezka, R., Laage, R., Brosig, B. & Langosch, D. A heptad motif of leucine residues found in membrane proteins can drive self-assembly of artificial transmembrane segments. J. Biol. Chem. 274, 9265–9270 (1999).
Kern, J. et al. Purification, characterisation and crystallisation of photosystem II from Thermosynechococcus elongatus cultivated in a new type of photobioreactor. Biochim. Biophys. Acta 1706, 147–157 (2005).
Krivanek, R., Kern, J., Zouni, A., Dau, H. & Haumann, M. Spare quinones in the QB cavity of crystallized photosystem II from Thermosynechococcus elongates. Biochim. Biophys. Acta 1767, 520–527 (2007).
Kaminskaya, O., Shuvalov, V.A. & Renger, G. Evidence for a novel quinone-binding site in the photosystem II (PS II) complex that regulates the redox potential of cytochrome b559. Biochemistry 46, 1091–1105 (2007).
Ishida, N. et al. Biosynthetic exchange of bromide for chloride and strontium for calcium in the photosystem II oxygen-evolving enzymes. J. Biol. Chem. 283, 13330–13340 (2008).
Yocum, C.F. The calcium and chloride requirements of the O2 evolving complex. Coord. Chem. Rev. 252, 296–305 (2008).
Mueller-Dieckmann, C. et al. On the routine use of soft X-rays in macromolecular crystallography. Part IV. Efficient determination of anomalous substructures in biomacromolecules using longer X-ray wavelengths. Acta Crystallogr. D Biol. Crystallogr. 63, 366–380 (2007).
Murray, J.W. & Barber, J. Identification of a calcium-binding site in the PsbO protein of photosystem II. Biochemistry 45, 4128–4130 (2006).
Murray, J.W. & Barber, J. Structural characteristics of channels and pathways in photosystem II including the identification of an oxygen channel. J. Struct. Biol. 159, 228–237 (2007).
Petrek, M. et al. CAVER: a new tool to explore routes from protein clefts, pockets and cavities. BMC Bioinformatics 7, 316 (2006).
Kawakami, K., Iwai, M., Ikeuchi, M., Kamiya, N. & Shen, J.R. Location of PsbY in oxygen-evolving photosystem II revealed by mutagenesis and X-ray crystallography. FEBS Lett. 581, 4983–4987 (2007).
Iwai, M., Suzuki, T., Dohmae, N., Inoue, Y. & Ikeuchi, M. Absence of the PsbZ subunit prevents association of PsbK and Ycf12 with the PSII complex in the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. Plant Cell Physiol. 48, 1758–1763 (2007).
Jordan, P. et al. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411, 909–917 (2001).
Yamashita, E., Zhang, H. & Cramer, W.A. Structure of the cytochrome b6f complex: quinone analogue inhibitors as ligands of heme cn . J. Mol. Biol. 370, 39–52 (2007).
Stroebel, D., Choquet, Y., Popot, J.L. & Picot, D. An atypical haem in the cytochrome b6f complex. Nature 426, 413–418 (2003).
Jones, M.R. Lipids in photosynthetic reaction centres: structural roles and functional holes. Prog. Lipid Res. 46, 56–87 (2007).
Lancaster, C.R., Hunte, C., Kelley, J., III, Trumpower, B.L. & Ditchfield, R. A comparison of stigmatellin conformations, free and bound to the photosynthetic reaction center and the cytochrome bc1 complex. J. Mol. Biol. 368, 197–208 (2007).
Shinzawa-Itoh, K. et al. Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 26, 1713–1725 (2007).
Vass, I. & Aro, E.M. Photoinhibition of photosynthetic electron transport. in Primary Processes of Photosynthesis: Basic Principles and Apparatus Vol.1 (ed. Renger, G.) 393–425 (Royal Society of Chemistry, Cambridge, 2008).
Palsdottir, H. & Hunte, C. Lipids in membrane protein structures. Biochim. Biophys. Acta 1666, 2–18 (2004).
Rokka, A., Suorsa, M., Saleem, A., Battchikova, N. & Aro, E.M. Synthesis and assembly of thylakoid protein complexes. Multiple assembly steps of photosystem II. Biochem. J. 388, 159–168 (2005).
Nishiyama, Y., Allakhverdiev, S.I. & Murata, N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta 1757, 742–749 (2006).
Kruse, O. et al. Phosphatidylglycerol is involved in the dimerization of photosystem II. J. Biol. Chem. 275, 6509–6514 (2000).
Liu, Z. et al. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428, 287–292 (2004).
Standfuss, J., Terwisscha van Scheltinga, A.C., Lamborghini, M. & Kuehlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J. 24, 919–928 (2005).
Laczkó-Dobos, H. et al. Role of phosphatidylglycerol in the function and assembly of photosystem II reaction center, studied in a cdsA-inactivated PAL mutant strain of Synechocystis sp. PCC6803 that lacks phycobilisomes. Biochim. Biophys. Acta 1777, 1184–1194 (2008).
Gombos, Z. et al. Phosphatidylglycerol requirement for the function of electron acceptor plastoquinone QB in the photosystem II reaction center. Biochemistry 41, 3796–3802 (2002).
Barber, J., Morris, E.P. & da Fonseca, P.C. Interaction of the allophycocyanin core complex with photosystem II. Photochem. Photobiol. Sci. 2, 536–541 (2003).
Murray, J.W., Maghlaoui, K. & Barber, J. The structure of allophycocyanin from Thermosynechococcus elongatus at 3.5 Å resolution. Acta Crystallogr. F63, 998–1002 (2007).
Bondarava, N. et al. Evidence that cytochrome b559 mediates the oxidation of reduced plastoquinone in the dark. J. Biol. Chem. 278, 13554–13560 (2003).
Gao, X. et al. Structural basis for the quinone reduction in the bc1 complex: a comparative analysis of crystal structures of mitochondrial cytochrome bc1 with bound substrate and inhibitors at the Qi site. Biochemistry 42, 9067–9080 (2003).
Yano, J. & Yachandra, V.K. Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster from X-ray spectroscopy. Inorg. Chem. 47, 1711–1726 (2008).
Yano, J. et al. X-ray damage to the Mn4Ca complex in single crystals of photosystem II: a case study for metalloprotein crystallography. Proc. Natl. Acad. Sci. USA 102, 12047–12052 (2005).
Grabolle, M., Haumann, M., Müeller, C., Liebisch, P. & Dau, H. Rapid loss of structural motifs in the manganese complex of oxygenic photosynthesis by X-ray irradiation at 10–300 K. J. Biol. Chem. 281, 4580–4588 (2006).
Yano, J. et al. Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster. Science 314, 821–825 (2006).
Olesen, K. & Andreasson, L.E. The function of the chloride ion in photosynthetic oxygen evolution. Biochemistry 42, 2025–2035 (2003).
Lindberg, K. & Andreasson, L.E. A one-site, two-state model for the binding of anions in photosystem II. Biochemistry 35, 14259–14267 (1996).
Haumann, M. et al. Bromide does not bind to the Mn4Ca complex in its S1 state in Cl−-depleted and Br−-reconstituted oxygen-evolving photosystem II: evidence from X-ray absorption spectroscopy at the Br K-edge. Biochemistry 45, 13101–13107 (2006).
Debus, R.J., Strickler, M.A., Walker, L.M. & Hillier, W. No evidence from FTIR difference spectroscopy that aspartate-170 of the D1 polypeptide ligates a manganese ion that undergoes oxidation during the S0 to S1, S1 to S2, or S2 to S3 transitions in photosystem II. Biochemistry 44, 1367–1374 (2005).
Debus, R.J. Protein ligation of the photosynthetic oxygen-evolving center. Coord. Chem. Rev. 252, 244–258 (2008).
Murray, J.W. et al. X-Ray crystallography identifies two chloride binding sites in the oxygen evolving centre of photosystem II. Energy. Environ. Sci. 1, 161–166 (2008).
Ho, F.M. & Styring, S. Access channels and methanol binding site to the CaMn4 cluster in photosystem II based on solvent accessibility simulations, with implications for substrate water access. Biochim. Biophys. Acta 1777, 140–153 (2008).
Chu, H.A., Nguyen, A.P. & Debus, R.J. Amino acid residues that influence the binding of manganese or calcium to photosystem II. 1. The lumenal interhelical domains of the D1 polypeptide. Biochemistry 34, 5839–5858 (1995).
Ishikita, H., Saenger, W., Loll, B., Biesiadka, J. & Knapp, E.W. Energetics of a possible proton exit pathway for water oxidation in photosystem II. Biochemistry 45, 2063–2071 (2006).
Svensson-Ek, M. et al. The X-ray crystal structures of wild-type and EQ(I-286) mutant cytochrome c oxidases from Rhodobacter sphaeroides. J. Mol. Biol. 321, 329–339 (2002).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Brünger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Kashino, Y., Koike, H. & Satoh, K. An improved sodium dodecyl sulfate-polyacrylamide gel electrophoresis system for the analysis of membrane protein complexes. Electrophoresis 22, 1004–1007 (2001).
Acknowledgements
The authors are grateful to the Deutsche Forschungsgemeinschaft for support within the framework of Sfb 498 (projects A4, C7). We also acknowledge W. Kabsch for help with XDS, W. Schröder for help with protein sequencing, P. Franke and C. Weise for MS data collection and J. Biesiadka for cooperation. We thank F. Müh, G. Renger, R. Clarke, V. Yachandra and K. Sauer for discussion and careful reading of the manuscript. Beam time and support at ESRF (Grenoble), SLS (Villigen) and BESSY (Berlin) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
M.B., J.K. and A.Z. purified and crystallized the protein; A. Guskov, M.B., J.K. and A. Gabdulkhakov collected diffraction data; A. Guskov and A. Gabdulkhakov did structure determination and model building; A. Gabdulkhakov calculated channels; A. Guskov, J.K., M.B., A. Gabdulkhakov, A.Z. and W.S. designed experiments, analyzed data and prepared the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Tables 1‐4 and Supplementary Discussion (PDF 794 kb)
Rights and permissions
About this article
Cite this article
Guskov, A., Kern, J., Gabdulkhakov, A. et al. Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nat Struct Mol Biol 16, 334–342 (2009). https://doi.org/10.1038/nsmb.1559
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1559
This article is cited by
-
The photosystem-II repair cycle: updates and open questions
Planta (2024)
-
Influence of the antiseptic octenidine on spectral characteristics and energy migration processes in photosystem II core complexes
Photosynthesis Research (2023)
-
Solar energy conversion by photosystem II: principles and structures
Photosynthesis Research (2023)
-
Evolutionary diversity of proton and water channels on the oxidizing side of photosystem II and their relevance to function
Photosynthesis Research (2023)
-
High-Field EPR Investigation and Detailed Modeling of the Magnetoanisotropy Tensor of an Unusual Mixed-Valent MnIV2MnIII2MnII Cluster
Applied Magnetic Resonance (2023)