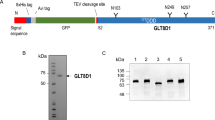
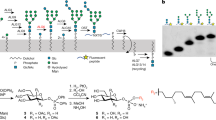
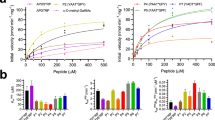
Abstract
N-Acetylglucosamine (O-GlcNAc) modification of proteins provides a mechanism for the control of diverse cellular processes through a dynamic interplay with phosphorylation. UDP-GlcNAc:polypeptidyl transferase (OGT) catalyzes O-GlcNAc addition. The structure of an intact OGT homolog and kinetic analysis of human OGT variants reveal a contiguous superhelical groove that directs substrates to the active site.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Hart, G.W., Housley, M.P. & Slawson, C. Nature 446, 1017–1022 (2007).
Wells, L., Vosseller, K. & Hart, G.W. Science 291, 2376–2378 (2001).
Vosseller, K., Wells, L., Lane, M.D. & Hart, G.W. Proc. Natl. Acad. Sci. USA 99, 5313–5318 (2002).
Kreppel, L.K., Blomberg, M.A. & Hart, G.W. J. Biol. Chem. 272, 9308–9315 (1997).
Lubas, W.A. & Hanover, J.A. J. Biol. Chem. 275, 10983–10988 (2000).
Lazarus, B.D., Roos, M.D. & Hanover, J.A. J. Biol. Chem. 280, 35537–35544 (2005).
Gross, B.J., Kraybill, B.C. & Walker, S. J. Am. Chem. Soc. 127, 14588–14589 (2005).
Lazarus, B.D., Love, D.C. & Hanover, J.A. Glycobiology 16, 415–421 (2006).
Jinek, M. et al. Nat. Struct. Mol. Biol. 11, 1001–1007 (2004).
Lairson, L., Henrissat, B., Davies, G.J. & Withers, S.G. Annu. Rev. Biochem. published online, doi:10.1146/annurev.biochem.76.061005.092322 (14 April 2008).
Yang, X.Y. et al. Nature 451, 964–969 (2008).
Conti, E. & Kuriyan, J. Structure 8, 329–338 (2000).
Acknowledgements
This work was funded by the UK Biotechnology and Biological Sciences Research Council and the the Canadian Institutes of Health Research. G.J.D. is a Royal Society/Wolfson research Merit Award recipient. D.J.V. is a Canada Research Chair in chemical glycobiology and a fellow of the Michael Smith Foundation for Health Research (MSFHR). M.S.M. is a scholar of the MSFHR.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Tables 1 and 2, and Supplementary Methods (PDF 5615 kb)
Supplementary Movie 1
Rotating view of the XcOGT (cyan)/Human TPR domain (gold) model with an importin-derived peptide in yellow and UDP in red. (AVI 8436 kb)
Rights and permissions
About this article
Cite this article
Martinez-Fleites, C., Macauley, M., He, Y. et al. Structure of an O-GlcNAc transferase homolog provides insight into intracellular glycosylation. Nat Struct Mol Biol 15, 764–765 (2008). https://doi.org/10.1038/nsmb.1443
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1443
This article is cited by
-
FpOGT is required for fungal growth, stress response, and virulence of Fusarium proliferatum by affecting the expression of glucokinase and other glucose metabolism-related genes
Phytopathology Research (2024)
-
Molecular basis for bacterial N-glycosylation by a soluble HMW1C-like N-glycosyltransferase
Nature Communications (2023)
-
O-GlcNAcylation of MORC2 at threonine 556 by OGT couples TGF-β signaling to breast cancer progression
Cell Death & Differentiation (2022)
-
RNA binding motif protein 3 (RBM3) promotes protein kinase B (AKT) activation to enhance glucose metabolism and reduce apoptosis in skeletal muscle of mice under acute cold exposure
Cell Stress and Chaperones (2022)
-
The Arabidopsis O-fucosyltransferase SPINDLY activates nuclear growth repressor DELLA
Nature Chemical Biology (2017)