Abstract
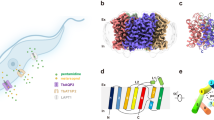
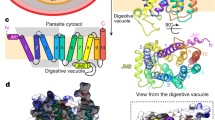
The 2.05-Å resolution structure of the aquaglyceroporin from the malarial parasite Plasmodium falciparum (PfAQP), a protein important in the parasite's life cycle, has been solved. The structure provides key evidence for the basis of water versus glycerol selectivity in aquaporin family members. Unlike its closest homolog of known structure, GlpF, the channel conducts both glycerol and water at high rates, framing the question of what determines high water conductance in aquaporin channels. The universally conserved arginine in the selectivity filter is constrained by only two hydrogen bonds in GlpF, whereas there are three in all water-selective aquaporins and in PfAQP. The decreased cost of dehydrating the triply-satisfied arginine cation may provide the basis for high water conductance. The two Asn-Pro-Ala (NPA) regions of PfAQP, which bear rare substitutions to Asn-Leu-Ala (NLA) and Asn-Pro-Ser (NPS), participate in preserving the orientation of the selectivity filter asparagines in the center of the channel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Hansen, M., Kun, J.F., Schultz, J.E. & Beitz, E. A single, bi-functional aquaglyceroporin in blood-stage Plasmodium falciparum malaria parasites. J. Biol. Chem. 277, 4874–4882 (2002).
Vial, H.J. & Ancelin, M.L. Malarial lipids. An overview. Subcell. Biochem. 18, 259–306 (1992).
Vial, H.J., Ancelin, M.L., Thuet, M.J. & Philippot, J.R. Phospholipid metabolism in Plasmodium-infected erythrocytes: guidelines for further studies using radioactive precursor incorporation. Parasitology 98, 351–357 (1989).
Beitz, E., Pavlovic-Djuranovic, S., Yasui, M., Agre, P. & Schultz, J.E. Molecular dissection of water and glycerol permeability of the aquaglyceroporin from Plasmodium falciparum by mutational analysis. Proc. Natl. Acad. Sci. USA 101, 1153–1158 (2004).
Promeneur, D. et al. Aquaglyceroporin PbAQP during intraerythrocytic development of the malaria parasite Plasmodium berghei. Proc. Natl. Acad. Sci. USA 104, 2211–2216 (2007).
Liu, Y. et al. Aquaporin 9 is the major pathway for glycerol uptake by mouse erythrocytes, with implications for malarial virulence. Proc. Natl. Acad. Sci. USA 104, 12560–12564 (2007).
Gardner, M.J. et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419, 498–511 (2002).
Jayaraj, S., Reid, R. & Santi, D.V. GeMS: an advanced software package for designing synthetic genes. Nucleic Acids Res. 33, 3011–3016 (2005).
Fu, D. et al. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290, 481–486 (2000).
Sui, H., Han, B.G., Lee, J.K., Walian, P. & Jap, B.K. Structural basis of water-specific transport through the AQP1 water channel. Nature 414, 872–878 (2001).
Fujiyoshi, Y. et al. Structure and function of water channels. Curr. Opin. Struct. Biol. 12, 509–515 (2002).
Kozono, D. et al. Functional expression and characterization of an archaeal aquaporin. AqpM from Methanothermobacter marburgensis. J. Biol. Chem. 278, 10649–10656 (2003).
Fu, D., Libson, A. & Stroud, R. The structure of GlpF, a glycerol conducting channel. Novartis Found. Symp. 245, 51–61; Discussion 61–5, 165–8 (2002).
Wang, Y., Schulten, K. & Tajkhorshid, E. What makes an aquaporin a glycerol channel? A comparative study of AqpZ and GlpF. Structure 13, 1107–1118 (2005).
Jensen, M.O., Park, S., Tajkhorshid, E. & Schulten, K. Energetics of glycerol conduction through aquaglyceroporin GlpF. Proc. Natl. Acad. Sci. USA 99, 6731–6736 (2002).
Lee, J.K. et al. Structural basis for conductance by the archaeal aquaporin AqpM at 1.68 Å. Proc. Natl. Acad. Sci. USA 102, 18932–18937 (2005).
Savage, D.F., Egea, P.F., Robles-Colmenares, Y., O'Connell, J.D. III & Stroud, R.M. Architecture and selectivity in aquaporins: 2.5 X-ray structure of aquaporin Z. PLoS Biol. 1, e72 (2003).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
McCoy, A.J., Grosse-Kunstleve, R.W., Storoni, L.C. & Read, R.J. Likelihood-enhanced fast translation functions. Acta Crystallogr. D Biol. Crystallogr. 61, 458–464 (2005).
Perrakis, A., Morris, R. & Lamzin, V.S. Automated protein model building combined with iterative structure refinement. Nat. Struct. Biol. 6, 458–463 (1999).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Winn, M.D., Isupov, M.N. & Murshudov, G.N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D Biol. Crystallogr. 57, 122–133 (2001).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Davis, I.W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
Smart, O.S., Goodfellow, J.M. & Wallace, B.A. The pore dimensions of gramicidin A. Biophys. J. 65, 2455–2460 (1993).
Acknowledgements
Research was supported by the US National Institutes of Health (NIH) grant GM24485 to R.M.S. and by the NIH Roadmap center grant P50 GM073210. The authors thank the reviewers for their helpful suggestions. The authors thank F.A. Hays, P. Egea, J. Lee and D. Savage in the Stroud laboratory for helpful advice throughout the project and with preparation of the manuscript. The authors thank T.P. Kearney III and M. Miller for advice on the manuscript. We thank J. Holton for his assistance at the Advanced Light Source (ALS) beamline 8.3.1, supported by NIH grant GM074929 (R.M.S.).
Author information
Authors and Affiliations
Contributions
Z.E.R.N., J.O. III, Y.R.-C. and S.K. carried out experiments; Z.E.R.N. collected diffraction data and determined the structure; R.M.S. and L.J.M. supervised the research; Z.E.R.N. and R.M.S. wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 (PDF 11070 kb)
Rights and permissions
About this article
Cite this article
Newby, Z., O'Connell III, J., Robles-Colmenares, Y. et al. Crystal structure of the aquaglyceroporin PfAQP from the malarial parasite Plasmodium falciparum. Nat Struct Mol Biol 15, 619–625 (2008). https://doi.org/10.1038/nsmb.1431
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1431
This article is cited by
-
Glycerol biosynthetic pathway plays an essential role in proliferation and antioxidative defense in the human enteric protozoan parasite Entamoeba histolytica
Scientific Reports (2023)
-
Aquaporins Display a Diversity in their Substrates
The Journal of Membrane Biology (2023)
-
Structural basis for high selectivity of a rice silicon channel Lsi1
Nature Communications (2021)
-
Genome-wide identification and characterisation of Aquaporins in Nicotiana tabacum and their relationships with other Solanaceae species
BMC Plant Biology (2020)
-
Human adipose glycerol flux is regulated by a pH gate in AQP10
Nature Communications (2018)