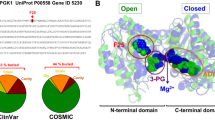
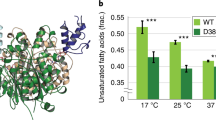
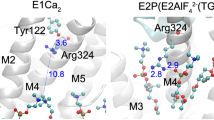
Abstract
Muscle acylphosphatase (AcP) is a small protein that folds very slowly with two-state behavior. The conformational stability and the rates of folding and unfolding have been determined for a number of mutants of AcP in order to characterize the structure of the folding transition state. The results show that the transition state is an expanded version of the native protein, where most of the native interactions are partially established. The transition state of AcP turns out to be remarkably similar in structure to that of the activation domain of procarboxypeptidase A2 (ADA2h), a protein having the same overall topology but sharing only 13% sequence identity with AcP. This suggests that transition states are conserved between proteins with the same native fold. Comparison of the rates of folding of AcP and four other proteins with the same topology, including ADA2h, supports the concept that the average distance in sequence between interacting residues (that is, the contact order) is an important determinant of the rate of protein folding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pastore, A., Saudek, V., Ramponi, G. & Williams, R.J.P. J. Mol. Biol. 224, 427–440 ( 1992).
Thunnissen, M.M.G.M., Taddei, N., Liguri, G., Ramponi, G. & Nordlund, P. Structure 5, 69– 79 (1997).
Taddei, N. et al. Eur. J. Biochem. 225, 811– 817 (1994).
van Nuland, N.A.J. et al. J. Mol. Biol. 283, 883– 891 (1998).
Chiti, F. et al. J. Mol. Biol. 283, 893– 903 (1998).
Chiti, F., et al. J. Biol. Chem. 274, 20151– 20158 (1999).
Matouschek, A., Kellis Jr, J.T., Serrano, L. & Fersht, A.R. Nature 340, 122–126 ( 1989).
Goldenberg, D.P., Frieden, R.W., Haack, J.A. & Morrison, T.B. Nature 338, 127–132 ( 1989).
Serrano, L., Matouschek, A. & Fersht, A.R. J. Mol. Biol. 224, 805– 818 (1992).
Itzhaki, L.S., Otzen, D.E. & Fersht, A.R. J. Mol. Biol. 254, 260– 288 (1995).
Milla, M.E., Brown, B.M., Waldburger, C.D. & Sauer, R.T. P22 Biochemistry 34, 13914– 13919 (1995).
Sosnick, T.R., Jackson, S., Wilk, R.R., Englander, S.W. & DeGrado, W.F. Proteins 24, 427– 432 (1996).
Viguera, A.R., Wilmanns, M. & Serrano, L Nature Struct. Biol. 3, 874– 880 (1996).
Burton, R.E., Huang, G.S., Daugherty, M.A., Calderone, T.L. & Oas, T.G. Nature Struct. Biol. 4, 305–310 (1997).
Lopez-Hernandez, E. & Serrano, L. Folding & Design 1, 43–55 ( 1996).
Grantcharova, V.P., Riddle, D.S., Santiago, J.V. & Baker, D. Nature Struct. Biol. 5, 714–720 (1998).
Villegas, V., Martinez, J.C., Aviles, F.X. & Serrano, L. J. Mol. Biol. 283, 1027–1036 (1998).
Jackson, S.E. Folding & Design 3, R81–R91 (1998).
Fersht, A.R., Itzhaki, L.S., ElMasry, N.F., Matthews, J.M., Otzen D.E. Proc. Natl. Acad. Sci. USA 91, 10426–10429 (1994).
Martinez, J.C., Pisabarro, M.T. & Serrano, L. Nature Struct. Biol. 5, 721– 729 (1998).
Abkevich, V.I., Gutin, A.M. & Shakhnovich, E.I. Biochemistry 33, 10026– 10036 (1994).
Plaxco, K.W., Simons, K.T. & Baker, D. J. Mol. Biol. 277, 985– 994 (1998).
Villegas, V. et al. Biochemistry 34, 15105– 15110 (1995).
van Nuland, N.A.J. et al. Biochemistry 37, 622– 637 (1998).
Silow, M. & Oliveberg, M. Biochemistry 36, 7633–7636 (1997).
Aronsson, G., Brorsson, A.-C., Sahlman, L. & Jonsson, B.-H. FEBS Lett. 411, 359–364 (1997).
Taddei, N. et al. Biochemistry 35, 7077– 7083 (1996).
Santoro, M.M. & Bolen, D.W. Biochemistry 27, 8063–8068 (1988).
Jackson, S.E. & Fersht, A.R. Biochemistry 30, 10428–10435 (1991).
Acknowledgements
We are grateful to C. Capanni, A. Giacobini, E. Giannoni and E. Chung for their technical support. We are also indebted to K. Plaxco for his help in the determination of the relative contact order values, to L. Serrano and E. Shakhnovich for very useful discussions and to G. Ramponi for his support. F.C. is supported in part by a short-term grant from the Società Italiana di Biochimica. This is a contribution from the Oxford Centre for Molecular Sciences, which is funded by the Biotechnology and Biological Sciences Research Council, the Engineering and Physical Sciences Research Council and the Medical Research Council. The work has also been supported by funds from the Consiglio Nazionale delle Ricerche, from the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (Project Biotechnology) and from the European Community. The research of C.M.D. is supported in part by an International Research Scholars award from the Howard Hughes Medical Institute and by a programme grant from the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiti, F., Taddei, N., White, P. et al. Mutational analysis of acylphosphatase suggests the importance of topology and contact order in protein folding. Nat Struct Mol Biol 6, 1005–1009 (1999). https://doi.org/10.1038/14890
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/14890
This article is cited by
-
Rheostat positions: A new classification of protein positions relevant to pharmacogenomics
Medicinal Chemistry Research (2020)
-
Network Connectivity, Centrality and Fragmentation in the Greek-Key Protein Topology
The Protein Journal (2019)
-
Types and effects of protein variations
Human Genetics (2015)
-
Sequence analysis on the information of folding initiation segments in ferredoxin-like fold proteins
BMC Structural Biology (2014)
-
Statistical analyses of protein folding rates from the view of quantum transition
Science China Life Sciences (2014)