Abstract
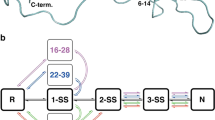
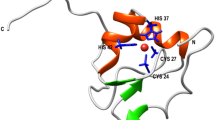
A previously unidentified intermediate has been detected in the early stages of the oxidative folding of bovine pancreatic trypsin inhibitor (BPTI). The intermediate contains one disulphide bond between residues 14 and 38 and is denoted [14–38]. The 14–38 disulphide bond is also found in native BPTI. Although the other native one-disulphide intermediates, [30–51] and [5–55], are thermodynamically more stable, [14–38] can be populated substantially at the early stages of BPTI folding. Moreover, initial characterization of the kinetic properties of this intermediate strongly suggest that a substantial fraction of BPTI molecules fold by way of the [14–38] intermediate. Our results emphasize the importance of native-like tendencies in protein folding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Creighton, T.E. Renaturation of the reduced bovine pancreatic trypsin inhibitor. J. molec. Biol. 87, 563–577 (1974).
Creighton, T.E. Conformational restrictions on the pathway of folding and unfolding of BPTI. J. molec. Biol. 113, 275–293 (1977).
Creighton, T.E. Energetics of folding and unfolding of pancreatic trypsin inhibitor. J. molec. Biol. 113, 295–312 (1977).
Creighton, T.E. Effects of urea and guanidine. HCI on the folding and unfolding of pancreatic trypsin inhibitor. J. molec. Biol. 113, 313–328 (1977).
Creighton, T.E. & Goldenberg, D.P. Kinetic role of a meta-stable native like two disulphide species in the folding transition of bovine pancreatic trypsin inhibitor. J. molec. Biol. 179, 497–526 (1984).
Goldenberg, D.P. Kinetic analysis of the folding and unfolding of a mutant form of bovine pancreatic trypsin inhibitor lacking the cysteine-14 and -38 thiols. Biochemistry 27, 2481–2489 (1988).
Weissman, J.S. & Kim, P.S. Reexamination of the folding of BPTI: predominance of native intermediates. Science 253, 1386–1393 (1991).
Weissman, J.S. & Kim, P.S. Kinetic role of nonnative species in the folding of bovine pancreatic trypsin inhibitor. Proc. natn. Acad. Sci. U.S.A. 89, 9900–9904 (1992).
Weissman, J.S. & Kim, P.S. The pro region of BPTI facilitates folding. Cell 71, 841–851 (1992).
Weissman, J.S. & Kim, P.S. Efficient catalysis of disulphide bond rearrangements by protein disulphide isomerase. Nature 365, 185–188 (1993).
Creighton, T.E. Reactivities of cysteine residues of the reduced pancreatic trypsin inhibitor. J. molec. Biol. 96, 777–782 (1975).
Darby, N.J. & Creighton, T.E. Dissecting the disulphide-coupled folding pathway of bovine pancreatic trypsin inhibitor. J. molec. Biol. 232, 873–896 (1993).
States, D.J., Creighton, T.E., Dobson, C.M. & Karplus, M. Conformations of intermediates in the folding of pancreatic trypsin inhibitor. J. molec. Biol. 195, 731–739 (1987).
Oas, T.G. & Kim, P.S. A peptide model of a protein folding intermediate.Nature 336, 42–48 (1988).
Staley, J.P. & Kim, P.S. Role of a subdomain in the folding of bovine pancreatic trypsin inhibitor. Nature 344, 685–688 (1990).
van Mierlo, C.P.M., Darby, N.J., Neuhaus, D. & Creighton, T.E. . Two-dimensional 1H nuclear magnetic resonance study of the [5-55] single-disulphide folding intermediate of bovine pancreatic trypsin inhibitor. J. molec. Biol. 222, 373–390 (1991).
Staley, J.P. & Kim, P.S. Complete folding of BPTI with only a single disulphide bond. Proc. natn. Acad. Sci. U.S.A. 89, 1519–1523 (1992).
van Mierlo, C.P.M., Darby, N.J. & Creighton, T.E. . The partially folded conformation of the [30-51] intermediate in the disulphide folding pathway of bovine pancreatic trypsin inhibitor. Proc. natn. Acad. Sci. U.S.A. 89, 6775–6779 (1992).
van Mierlo, C.P.M., Darby, N.J., Keeler, J., Neuhaus, D. & Creighton, T.E. . Partially folded conformation of the [30-51] intermediate in the disulphide folding pathway of bovine pancreatic trypsin inhibitor. J. molec. Biol. 229, 1125–1146 (1993).
Staley, J.P. & Kim, P.S. Formation of a native-like subdomain in a partially folded intermediate of BPTI. Prot. Sci. 10, 1822–1832 (1994).
Goto, Y. & Hamaguchi, K. Formation of the intrachain disulphide bond in the constant fragment of the immunoglobulin light chain. J. molec. Biol. 146, 321–340 (1981).
Goldenberg, D.P. Native and non-native intermediates on the BPTI folding pathway. Trends biochem. Sci. 17, 257–261 (1992).
Ferrer, M., Barany, G. & Woodward, C. Partially folded molten globule and molten coil states of bovine pancreatic trypsin inhibitor. Nature struct. Biology 2, 211–217 (1995).
Deisenhofer, J. & Steigemann, W. Crystallographic refinement of the structure of bovine pancreatic trypsin inhibitor at 1.5Å resolution. Acta Crystallogr. B31, 238–250 (1975).
Wlodawer, A., Walter, J., Huber, R. & Sjölin, L. Structure of bovine pancreatic trypsin inhibitor. J. molec. Biol. 180, 301–329 (1984).
Berndt, K.D., Guntert, P., Orbons, L.P. & Wüthrich, K. Determination of a high-quality nuclear magnetic resonance solution structure of the bovine pancreatic trypsin inhibitor and comparison with three crystal structures. J. molec. Biol. 227, 757–775 (1992).
Lee, B. & Richards, F.M. The interpretation of protein structures: estimation of static accessibility. J. molec. Biol. 55, 379–400 (1971).
Marks, C.B., Naderi, H., Kosen, P.A., Kuntz, I. & Anderson, S. Mutants of bovine pancreatic trypsin inhibitor lacking cysteines 14 and 38 can fold properly. Science 235, 1370–1372 (1987).
Zhang, J.-X. & Goldenberg, D.P. Amino acid replacement that eliminates kinetic traps in the folding pathway of pancreatic trypsin inhibitor. Biochemistry 32, 14075–14081 (1993).
Creighton, T.E. Disulphide bonds as probes of protein folding pathways. Meth. Enzymol. 131, 83–107 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dadlez, M., Kim, P. A third native one-disulphide intermediate in the folding of bovine pancreatic trypsin inhibitor. Nat Struct Mol Biol 2, 674–679 (1995). https://doi.org/10.1038/nsb0895-674
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0895-674
This article is cited by
-
A kinetic explanation for the rearrangement pathway of BPTI folding
Nature Structural & Molecular Biology (1995)