Abstract
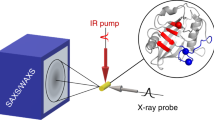
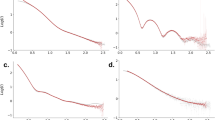
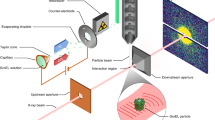
We have used small angle X-ray scattering (SAXS) to monitor changes in the overall size and shape of the Tetrahymena ribozyme as it folds. The native ribozyme, formed in the presence of Mg2+, is much more compact and globular than the ensemble of unfolded conformations. Time-resolved measurements show that most of the compaction occurs at least 20-fold faster than the overall folding to the native state, suggesting that a compact intermediate or family of intermediates is formed early and then rearranges in the slow steps that limit the overall folding rate. These results lead to a kinetic folding model in which an initial ‘electrostatic collapse’ of the RNA is followed by slower rearrangements of elements that are initially mispositioned.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Treiber, D.K. & Williamson, J.R. Curr. Opin. Struct. Biol. 9, 339–345 (1999).
Ferre-D'Amare, A.R. & Doudna, J.A. Annu. Rev. Biophys. Biomol. Struct. 28, 57–73 (1999).
Latham, J.A. & Cech, T.R. Science 245, 276–282 (1989).
Golden, B.L., Gooding, A.R., Podell, E.R. & Cech, T.R. Science 282, 259–264 ( 1998).
Zarrinkar, P.P. & Williamson, J.R. Science 265, 918–924 (1994).
Sclavi, B., Sullivan, M., Chance, M.R., Brenowitz, M. & Woodson, S.A. Science 279, 1940–1943 (1998).
Glatter, O. & Kratky, O. Small angle X-ray scattering. (Academic Press, London; 1982).
Pan, J., Thirumalai, D. & Woodson, S.A. J. Mol. Biol. 273, 7– 13 (1997).
Treiber, D.K., Rook, M.S., Zarrinkar, P.P. & Williamson, J.R. Science 279, 1943–1946 (1998).
Zarrinkar, P.P. & Williamson, J.R. Nature Struct. Biol. 3, 432–438 ( 1996).
Lake, J.A. & Beeman, W.W. J. Mol. Biol. 31, 115–125 (1968).
Pilz, I., Kratky, O., Cramer, F., Haar, F.v.d. & Schlimme, E. Eur. J. Biochem. 15, 401– 409 (1970).
Kratky, O. & Pilz, I. Q. Rev. Biophys. 5, 481–537 (1972).
Plaxco, K.W., Millett, I.S., Segel, D.J., Doniach, S. & Baker, D. Nature Struct. Biol. 6 , 554–556 (1999).
Segel, D.J., et al. J. Mol. Biol. 288, 489– 499 (1999).
Pollack, L., et al. Proc. Natl. Acad. Sci. USA 96, 10115 –10117 (1999).
Celander, D.W. & Cech, T.R. Science 251, 401–407 (1991).
Herschlag, D. & Cech, T.R. Biochemistry 29, 10159–10171 (1990).
Grosshans, C.A. & Cech, T.R. Biochemistry 28, 6888–6894 (1989).
McConnell, T.S., Herschlag, D. & Cech, T.R. Biochemistry 36, 8293– 8303 (1997).
Murphy, F.L. & Cech, T.R. Biochemistry 32, 5291–5300 (1993).
Doherty, E.A. & Doudna, J.A. Biochemistry 36, 3159–3169 (1997).
Pan, T. & Sosnick, T.R. Nature Struct. Biol. 4, 931–938 (1997).
Russell, R. & Herschlag, D. J. Mol. Biol. 291, 1155–1167 (1999).
Rook, M.S., Treiber, D.K. & Williamson, J.R. J. Mol. Biol. 281, 609– 620 (1998).
Lehnert, V., Jaeger, L., Michel, F. & Westhof, E. Chem. Biol. 3, 993–1009 (1996).
Pan, J. & Woodson, S.A. J. Mol. Biol. 294, 955–965 (1999).
Herschlag, D. J. Biol. Chem. 270, 20871–20874 (1995).
Zaug, A.J., Grosshans, C.A. & Cech, T.R. Biochemistry 27, 8924– 8931 (1988).
Narlikar, G.J., Bartley, L.E., Khosla, M. & Herschlag, D. Biochemistry 38, 14192–14204 (1999).
Tsuruta, H., et al. J. Appl. Crystallogr. 31, 672– 682 (1998).
Semenyuk, A.V. & Svergun, D.I. J. Appl. Crystallogr. 24, 537–540 ( 1991).
Michel, F. & Westhof, E. J. Mol. Biol. 216, 585–610 (1990).
Acknowledgements
We thank H. Tsurata for help on beamline 4-2 and K. Hodgson for his support. This research was supported by an NIH grant to D.H. The US Department of Energy and the National Institutes of Health support SSRL. R.R. was supported by an NIH postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Russell, R., Millett, I., Doniach, S. et al. Small angle X-ray scattering reveals a compact intermediate in RNA folding . Nat Struct Mol Biol 7, 367–370 (2000). https://doi.org/10.1038/75132
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/75132
This article is cited by
-
Time-resolved structure investigation with small angle X-ray scattering using scanning techniques
Rendiconti Lincei (2011)