Abstract
The crystal structure of human p38 mitogen-activated protein (MAP) kinase in complex with a potent and highly specific pyridinyl-imidazole inhibitor has been determined at 2.0 Å resolution. The structure of the kinase, which is in its unphosphorylated state, is similar to that of the closely-related ERK2. The inhibitor molecule is bound in the ATP pocket. A hydrogen bond is made between the pyridyl nitrogen of the inhibitor and the main chain amido nitrogen of residue 109, analogous to the interaction from the N1 atom of ATP. The crystal structure provides possible explanations for the specificity of this class of inhibitors. Other protein kinase inhibitors may achieve their specificity through a similar mechanism. The structure also reveals a possible second binding site for this inhibitor, with currently unknown function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Seger, R. & Krebs, E.G. The MARK signaling cascade. FASEB J. 9, 726–735, (1995).
Cobb, M.H. & Goldsmith, E.J. How MAP kinases are regulated. J. Biol. Chem. 270, 14843–14846 (1995).
Johnson, L.N., Noble, M.E.M. & Owen, D.J. Active and inactive protein kinases: Structural basis for regulation. Cell 85, 149–158, (1996).
Taylor, S.S. & Radzio-Andzelm, E. Three protein kinase structures define a common motif. Structure, 2, 345–355 (1994).
Zheng, J. et al. Crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MgATP and peptide inhibitor. Biochem. 32, 2154–2161 (1993).
Bossemeyer, D., Engh, R.A., Kinzel, V., Ponstingl, H. & Huber, R. Phosphotransferase and substrate binding mechanism of the cAMP-dependent protein kinase catalytic subunit from porcine heart as deduced from the 2.0Å structure of the complex with Mn2+ adenylyl imidodiphosphate and inhibitor peptide PKI(5-24). EMBO J. 12, 849–859 (1993).
De Bondt, H.L. et al. Crystal structure of cyclin-dependent kinase 2. Nature 363, 595–602 (1993).
Zhang, F., Strand, A., Robbins, D., Cobb, M.H. & Goldsmith, E.J. Atomic structure of the MAP kinase ERK2 at 2.3Å resolution. Nature 367, 704–711 (1994).
Xu, R.-M., Carmel, G., Sweet, R.M., Kuret, J. & Cheng, X. Crystal structure of casein kinase-1, a phosphate-directed protein kinase. EMBO J. 14, 1015–1023 (1995).
Owen, D.J., Noble, M.E.M., Garman, E.F., Papageorgiou, A.C. & Johnson, L. N. Two structures of the catalytic domain of phosphorylase kinase: an active protein kinase complexed with substrate analogue and product. Structure 3, 467–482 (1995).
Han, J., Lee, J.-D., Bibbs, L. & Ulevitch, R.J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265, 808–811 (1994).
Rouse, J. et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78, 1027–1037 (1994).
Freshney, N.W. et al. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell 78, 1039–1049 (1994).
Lee, J.C. et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739–746 (1994).
Raingeaud, J. et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270, 7420–7426 (1995).
Lee, J.C. et al. Bicyclic imidazoles as a novel class of cytokine biosynthesis inhibitors. Ann. N.Y.Acad. Sci. 696 149–170 (1993).
Cuenda, A. et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364, 229–233 (1995).
Jiang, Y. et al. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β). J. Biol. Chem. 271, 17920–17926 (1996).
Li, Z., Jiang, Y., Ulevitch, R.J. & Han, J. The primary structure of p38γ: A new member of p38 group of MAP kinases. Biochem. Biophys. Res. Commun. 228, 334–340 (1996).
Pav, S. et al. Crystallization and preliminary crystallographic analysis of recombinant human p38 MAP kinase. Protein Sci. 6, 242–245 (1997).
Hendrickson, W.A. Determination of macromolecular structures from anomalous diffraction of synchrotron radiation. Science 254, 51–58 (1991).
Cox, S., Radzio-Andzelm, E. & Taylor, S.S. Domain movements in protein kinases. Curr. Opin. Struct. Biol. 4, 893–901 (1994).
Wilson, K.P. et al. Crystal structure of p38 mitogen-activated protein kinase. J. Biol. Chem. 271, 27696–27700 (1996).
Gallagher, T.F. et al. 2,4,5-triarylimidazole inhibitors of IL-1 biosynthesis. Bioorg. Med. Chem. Lett. 5, 1171–1176 (1995).
Boehm, J.C. et al. 1-substituted 4-aryl-5-pyridinylimidazoles: A new class of cytokine suppressive drugs with low 5-lipoxygenase and cyclooxygenase inhibitory potency. J. Med Chem. 39, 3929–3937 (1996).
Hanks, S.K., Quinn, A.M. & Hunter, T. The protein kinase family: Conserved features and deduced phylogeny of the catalytic domains. Science 241, 42–52 (1988).
Schulze-Gahmen, U. et al. Multiple modes of ligand recognition: Crystal structures of cyclin-dependent protein kinase 2 in complex with ATP and two inhibitors, olomoucine and isopentenyladenine. Proteins 22, 378–391 (1995).
De Azevedo, W.F., Jr. et al. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proc. Natl. Acad. Sci. USA 83, 2735–2740 (1996).
Xu, R.-M., Carmel, G., Kuret, J. & Cheng, X. Structural basis for selectivity of the isoquinoline sulfonamide family of protein kinase inhibitors. Proc. Natl. Acad. Sci. USA 93, 6308–6313 (1996).
Bourne, Y. et al. Crystal structure and mutational analysis of the human CDK2 kinase complex with cell cycle-regulatory protein CksHs1. Cell 84, 863–874 (1996).
Pines, J. Cell cycle: reaching for a role for the Cks proteins. Curr. Biol. 6, 1399–1402 (1996).
Fry, D.W. & Bridges, A.J. Inhibitors of protein tyrosine kinases. Curr. Opin. Biotech. 6, 662–667 (1995).
Hanke, J.H. et al. Discovery of a novel, potent, and src family-selective tyrosine kinase inhibitor. J. Biol. Chem. 271, 695–701 (1996).
Badger, A.M. et al. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J. Pharm. Exp. Therapeutics 279, 1453–1461 (1996).
Otwinowski, Z. In Data Collection and Processing (Sawyer, L., Isaacs, N. & Bailey, S., eds.) 56–62. (SERC Daresbury Laboratory) (1993).
Tong, L. Combined molecular replacement. Acta Cryst. A52, 782–784 (1996).
Tong, L. Replace: A suite of computer programs for molecular-replacement calculations. J. Appl. Crystallogr. 26, 748–751 (1993).
Brünger, A.T. The X-PLOR manual, version 3.0. Yale University, New Haven, CT. (1992).
Wang, B.C. Resolution of phase ambiguity in macromolecular crystallography. Meth. Enzymol. 115, 90–112 (1985).
Jones, T.A. A graphics model building and refinement system for macromolecules. J. Appl. Crystallogr. 11, 268–272 (1978).
Brünger, A.T. Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355, 472–475 (1992).
Carson, M. Ribbon models of macromolecules. J. Mol. Graphics, 5, 103–106 (1987).
Nicholls, A., Sharp, K.A. & Honig, B. Protein folding and association: insights from the interracial and thermodynamic properties of hydrocarbons. Proteins 11, 281–296 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tong, L., Pav, S., White, D. et al. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nat Struct Mol Biol 4, 311–316 (1997). https://doi.org/10.1038/nsb0497-311
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0497-311
This article is cited by
-
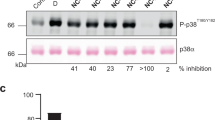
Characterization of p38α autophosphorylation inhibitors that target the non-canonical activation pathway
Nature Communications (2023)
-
Translocation of intracellular CD24 constitutes a triggering event for drug resistance in breast cancer
Scientific Reports (2021)
-
Comparative chemical array screening for p38γ/δ MAPK inhibitors using a single gatekeeper residue difference between p38α/β and p38γ/δ
Scientific Reports (2016)
-
Targeting substrate-site in Jak2 kinase prevents emergence of genetic resistance
Scientific Reports (2015)
-
Computational study of EGFR inhibition: molecular dynamics studies on the active and inactive protein conformations
Journal of Molecular Modeling (2013)