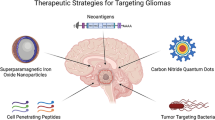
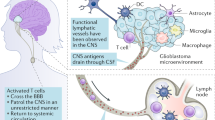
Key Points
-
Brain tumours represent a heterogeneous group of neoplasms, the most common being astrocytomas. Astrocytomas differ in their spectrum of behaviour, from those which are relatively benign to the most malignant, glioblastoma multiforme.
-
Due to their micro-environment within the central nervous system, brain tumours are well isolated from systemic circulation. This occurs because of the presence of the blood–brain barrier, the blood–cerebrospinal fluid barrier, and blood–tumour barrier. As such, systemic therapy of brain tumours has been unsuccessful.
-
The development of biodegradable polymers has changed the therapy of patients with brain tumours. Local and controlled delivery of antineoplastic agents such as Gliadel has overcome the inherent obstacles presented by the barriers of the central nervous system.
-
New advances in technology, gene therapy and immunology are likely to have a significant impact on the treatment of malignant brain tumours in the near future.
Abstract
Although previously considered untreatable, brain tumours no longer carry the same prognosis as they did even a decade ago. Recent advances in drug delivery to the central nervous system have not only bypassed physiological constraints such as the blood–brain barrier, but have, in fact, changed the course of treatment for patients with malignant brain tumours. The creation of targeted therapies, which spare normal tissue and destroy tumour cells, is changing the field of neuro-oncology. In this article, we review recent developments in the delivery of drugs to tumours of the central nervous system, discuss current trends and directions in the development of novel drugs and delivery systems, and present new and cutting-edge strategies for overcoming the challenges ahead.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mohan, D. S. et al. Outcome in elderly patients undergoing definitive surgery and radiation therapy for supratentorial glioblastoma multiforme at a tertiary care institution. Int. J. Radiat. Oncol. Biol. Phys. 42, 981–987 (1998).
Barker, F. G. et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 42, 709–920; discussion 720–723 (1998).
Roose, T., Netti, P. A., Munn, L. L., Boucher, Y. & Jain, R. K. Solid stress generated by spheroid growth estimated using a linear poroelasticity model small star, filled. Microvasc. Res. 66, 204–212 (2003).
Kornblith, P. L. & Walker, M. Chemotherapy for malignant gliomas. J. Neurosurg. 68, 1–17 (1988).
Albrecht, K. W. et al. High concentration of Daunorubicin and Daunorubicinol in human malignant astrocytomas after systemic administration of liposomal Daunorubicin. J. Neurooncol. 53, 267–271 (2001).
Koukourakis, M. I. et al. High intratumoural accumulation of stealth liposomal doxorubicin (Caelyx) in glioblastomas and in metastatic brain tumours. Br. J. Cancer 83, 1281–1286 (2000).
Fabel, K. et al. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer 92, 1936–1942 (2001).
Lippens, R. J. Liposomal daunorubicin (DaunoXome) in children with recurrent or progressive brain tumors. Pediatr. Hematol. Oncol. 16, 131–139 (1999).
Kreuter, J., Alyautdin, R. N., Kharkevich, D. A. & Ivanov, A. A. Passage of peptides through the blood–brain barrier with colloidal polymer particles (nanoparticles). Brain Res. 674, 171–174 (1995).
Schroeder, U., Sommerfeld, P., Ulrich, S. & Sabel, B. A. Nanoparticle technology for delivery of drugs across the blood–brain barrier. J. Pharm. Sci. 87, 1305–1307 (1998).
Alyautdin, R. N. et al. Delivery of loperamide across the blood–brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Pharm. Res. 14, 325–328 (1997).
Alyautdin, R. N. et al. Significant entry of tubocurarine into the brain of rats by adsorption to polysorbate 80-coated polybutylcyanoacrylate nanoparticles: an in situ brain perfusion study. J. Microencapsul. 15, 67–74 (1998).
Friese, A., Seiller, E., Quack, G., Lorenz, B. & Kreuter, J. Increase of the duration of the anticonvulsive activity of a novel NMDA receptor antagonist using poly(butylcyanoacrylate) nanoparticles as a parenteral controlled release system. Eur. J. Pharm. Biopharm. 49, 103–109 (2000).
Gulyaev, A. E. et al. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm. Res. 16, 1564–1569 (1999).
Rapoport, S. I. Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Expert Opin. Investig. Drugs. 10, 1809–1818 (2001).
Zylber-Katz, E. et al. Pharmacokinetics of methotrexate in cerebrospinal fluid and serum after osmotic blood-brain barrier disruption in patients with brain lymphoma. Clin. Pharmacol. Ther. 67, 631–641 (2000).
Rapoport, S. I. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell. Mol. Neurobiol. 20, 217–230 (2000).
Morikawa, N. et al. Dose-related increases in cerebrospinal fluid concentrations of methotrexate in a postoperative patient with glioblastoma. Ann. Pharmacother. 33, 952–956 (1999).
Kobrinsky, N. L. et al. Etoposide with or without mannitol for the treatment of recurrent or primarily unresponsive brain tumors: a Children's Cancer Group Study, CCG-9881. J. Neurooncol. 45, 47–54 (1999).
Prados, M. D. et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro-oncology 5, 96–103 (2003).
Boiardi, A. et al. Locally delivered chemotherapy and repeated surgery can improve survival in glioblastoma patients. Ital. J. Neurol. Sci. 20, 43–48 (1999).
Morantz, R. A., Kimler, B. F., Vats, T. S. & Henderson, S. D. Bleomycin and brain tumors. A review. J. Neurooncol. 1, 249–255 (1983).
Patchell, R. A. et al. A phase I trial of continuously infused intratumoral bleomycin for the treatment of recurrent glioblastoma multiforme. J. Neurooncol. 60, 37–42 (2002).
Voulgaris, S. et al. Intratumoral doxorubicin in patients with malignant brain gliomas. Am. J. Clin. Oncol. 25, 60–64 (2002).
Huang, Y., Hayes, R. L., Wertheim, S., Arbit, E. & Scheff, R. Treatment of refractory recurrent malignant glioma with adoptive cellular immunotherapy: a case report. Crit. Rev. Oncol. Hematol. 39, 17–23 (2001).
Boiardi, A. et al. Local immunotherapy (β-IFN) and systemic chemotherapy in primary glial tumors. Ital. J. Neurol. Sci. 12, 163–168 (1991).
Giussani, C. et al. Local intracerebral delivery of endogenous inhibitors by osmotic minipumps effectively suppresses glioma growth in vivo. Cancer Res. 63, 2499–2505 (2003).
Husain, S. R. & Puri, R. K. Interleukin-13 receptor-directed cytotoxin for malignant glioma therapy: from bench to bedside. J. Neurooncol. 65, 37–48 (2003).
Kunwar, S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir. Suppl. 88, 105–111 (2003).
Mardor, Y. et al. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res. 61, 4971–4973 (2001).
Lidar, Z. et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II study. J. Neurosurg. 100, 472–479 (2004).
Langer, R. & Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 263, 797–800 (1976). The original article describing polymers and their potential use in clinical applications.
Leong, K. W., Brott, B. C. & Langer, R. Bioerodible polyanhydrides as drug-carrier matrices. I: Characterization, degradation, and release characteristics. J. Biomed. Mater. Res. 19, 941–955 (1985).
Leong, K. W., D'Amore, P. D., Marletta, M. & Langer, R. Bioerodible polyanhydrides as drug-carrier matrices. II. Biocompatibility and chemical reactivity. J. Biomed. Mater. Res. 20, 51–64 (1986).
Domb, A., Bogdansky, S. & A., O. Controlled delivery of water soluble and hydrolytically unstable anti-cancer drugs for polymeric implants. Polymer Prepr. 32, 219–222 (1991).
Tabata, Y., Gutta, S. & Langer, R. Controlled delivery systems for proteins using polyanhydride microspheres. Pharm. Res. 10, 487–496 (1993).
Olivi, A. et al. Interstitial delivery of carboplatin via biodegradable polymers is effective against experimental glioma in the rat. Cancer Chemother. Pharmacol. 39, 90–96 (1996).
Menei, P. et al. Drug targeting into the central nervous system by stereotactic implantation of biodegradable microspheres. Neurosurgery 34, 1058–1064; discussion 1064 (1994).
Gref, R. et al. Biodegradable long-circulating polymeric nanospheres. Science 263, 1600–1603 (1994).
Gabizon, A. A. Liposomal anthracyclines. Hematol. Oncol. Clin. North. Am. 8, 431–450 (1994).
Golumbek, P. T. et al. Controlled release, biodegradable cytokine depots: a new approach in cancer vaccine design. Cancer Res. 53, 5841–5844 (1993).
Menei, P. et al. Stereotaxic implantation of 5-fluorouracil-releasing microspheres in malignant glioma. Cancer 100, 405–410 (2004).
Loo, T. L., Dion, R. L., Dixon, R. L. & Rall, D. P. The antitumor agent, 1,3-bis(2-choloethyl)-1-nitrosourea. J. Pharm. Sci. 55, 492–497 (1966).
Green, S. B. et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat. Rep. 67, 121–132 (1983).
Walker, M. D. et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N. Engl. J. Med. 303, 1323–1329 (1980).
Grossman, S. A. et al. The intracerebral distribution of BCNU delivered by surgically implanted biodegradable polymers. J. Neurosurg. 76, 640–647 (1992).
Yang, M. B., Tamargo, R. J. & Brem, H. Controlled delivery of 1,3-bis(2-chloroethyl)-1-nitrosourea from ethylene-vinyl acetate copolymer. Cancer Res. 49, 5103–5107 (1989).
Wu, M. P., Tamada, J. A., Brem, H. & Langer, R. In vivo versus in vitro degradation of controlled release polymers for intracranial surgical therapy. J. Biomed Mater. Res. 28, 387–395 (1994).
Tamargo, R. J. et al. Interstitial chemotherapy of the 9L gliosarcoma: controlled release polymers for drug delivery in the brain. Cancer Res. 53, 329–333 (1993).
Brem, H. et al. Biodegradable polymers for controlled delivery of chemotherapy with and without radiation therapy in the monkey brain. J. Neurosurg. 80, 283–290 (1994).
Brem, H. et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J. Neurosurg. 74, 441–446 (1991). One of the first papers to describe the efficacy of Gliadel against recurrent maligant brain tumors.
Brem, H. et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet 345, 1008–1012 (1995).
Westphal, M. et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncol. 5, 79–88 (2003).
Olivi, A. et al. Dose escalation of carmustine in surgically implanted polymers in patients with recurrent malignant glioma: a New Approaches to Brain Tumor Therapy CNS Consortium trial. J. Clin. Oncol. 21, 1845–1849 (2003).
Rhines, L. D. et al. O6-benzylguanine potentiates the antitumor effect of locally delivered carmustine against an intracranial rat glioma. Cancer Res. 60, 6307–6310 (2000).
Laws, E. R., Jr., Morris, A. M. & Maartens, N. Gliadel for pituitary adenomas and craniopharyngiomas. Neurosurgery 53, 255–269; discussion 259–260 (2003).
Ewend, M. G. et al. Local delivery of chemotherapy and concurrent external beam radiotherapy prolongs survival in metastatic brain tumor models. Cancer Res. 56, 5217–5223 (1996).
Ewend, M. G., Brem, S., Gilbert, M., Goodkin, R. & Penar, P. Treating single brain metastasis with resection, placement of BCNU-polymer wafers, and radiation therapy. Am. Assoc. Neurol. Surgeons Toronto, Canada, 24–26 April 2001.
Forsyth, P. et al. Phase II trial of docetaxel in patients with recurrent malignant glioma: a study of the National Cancer Institute of Canada Clinical Trials Group. Invest. New Drugs 14, 203–206 (1996).
Freilich, R. J., Seidman, A. D. & DeAngelis, L. M. Central nervous system progression of metastatic breast cancer in patients treated with paclitaxel. Cancer 76, 232–236 (1995).
Glantz, M. J., Chamberlain, M. C., Chang, S. M., Prados, M. D. & Cole, B. F. The role of paclitaxel in the treatment of primary and metastatic brain tumors. Semin. Radiat. Oncol. 9, 27–33 (1999).
Walter, K. A. et al. Interstitial taxol delivered from a biodegradable polymer implant against experimental malignant glioma. Cancer Res. 54, 2207–2212 (1994).
Li, K. W. et al. Polilactofate microspheres for Paclitaxel delivery to central nervous system malignancies. Clin. Cancer Res. 9, 3441–3447 (2003).
Sampath, P. et al. Camptothecin analogs in malignant gliomas: comparative analysis and characterization. J. Neurosurg. 98, 570–577 (2003).
Storm, P. B. et al. Polymer delivery of camptothecin against 9L gliosarcoma: release, distribution, and efficacy. J. Neurooncol. 56, 209–217 (2002).
Weingart, J. D., Thompson, R. C., Tyler, B., Colvin, O. M. & Brem, H. Local delivery of the topoisomerase I inhibitor camptothecin sodium prolongs survival in the rat intracranial 9L gliosarcoma model. Int. J. Cancer. 62, 605–609 (1995).
Tamargo, R. J., Bok, R. A. & Brem, H. Angiogenesis inhibition by minocycline. Cancer Res 51, 672–675 (1991).
Weingart, J. D., Sipos, E. P. & Brem, H. The role of minocycline in the treatment of intracranial 9L glioma. J. Neurosurg. 82, 635–640 (1995).
Santini, J. T., Jr. Cima, M. J. & Langer, R. A controlled-release microchip. Nature 397, 335–8 (1999). This article shows how a solid-state silicon chip could have the potential to deliver several drugs at different time points.
Richards Grayson, A. C. et al. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nature Mater. 2, 767–772 (2003). Describes a biodegradable polymeric microchip that allows release of several drugs in vivo.
Brooks, P. C., Clark, R. A. & Cheresh, D. A. Requirement of vascular integrin αvβ3 for angiogenesis. Science 264, 569–571 (1994).
Hynes, R. O. Integrins: a family of cell surface receptors. Cell 48, 549–554 (1987).
Howe, A., Aplin, A. E., Alahari, S. K. & Juliano, R. L. Integrin signaling and cell growth control. Curr. Opin. Cell Biol. 10, 220–231 (1988).
Longhurst, C. M. & Jennings, L. K. Integrin-mediated signal transduction. Cell. Mol. Life Sci. 54, 514–526 (1998).
Malik, R. K. Regulation of apoptosis by integrin receptors. J. Pediatr. Hematol. Oncol. 19, 541–545 (1997).
Scatena, M. et al. NF-κB mediates αvβ3 integrin-induced endothelial cell survival. J. Cell Biol. 141, 1083–1093 (1998).
Lesniak, M. S., Pai, S. I., Johns, D. & Pardoll, D. M. Targeted adenoviral gene delivery for gliomas. Am. Assoc. Neurol. Surgeons Chicago, Illinois, 3–5 April 2002.
Lang, F. F. et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J. Clin. Oncol. 21, 2508–2518 (2003).
Vecil, G. G. & Lang, F. F. Clinical trials of adenoviruses in brain tumors: a review of Ad-p53 and oncolytic adenoviruses. J. Neurooncol. 65, 237–246 (2003).
Glick, R. P., Lichtor, T., Kim, T. S., Ilangovan, S. & Cohen, E. P. Fibroblasts genetically engineered to secrete cytokines suppress tumor growth and induce antitumor immunity to a murine glioma in vivo. Neurosurgery 36, 548–555 (1995).
Glick, R. P., Lichtor, T., de Zoeten, E., Deshmukh, P. & Cohen, E. P. Prolongation of survival of mice with glioma treated with semiallogeneic fibroblasts secreting interleukin-2. Neurosurgery 45, 867–874 (1999).
Lichtor, T. et al. Application of interleukin-2-secreting syngeneic/allogeneic fibroblasts in the treatment of primary and metastatic brain tumors. Cancer Gene Ther. 9, 464–469 (2002).
Deshmukh, P., Glick, R. P., Lichtor, T., Moser, R. & Cohen, E. P. Immunogene therapy with interleukin-2-secreting fibroblasts for intracerebrally metastasizing breast cancer in mice. J. Neurosurg. 94, 287–292 (2001).
Thompson, R. C. et al. Systemic and local paracrine cytokine therapies using transduced tumor cells are synergistic in treating intracranial tumors. J. Immunother. Emphasis Tumor Immunol. 19, 405–413 (1996).
Lesniak, M. et al. Comparative analysis of paracrine immunotherapy in experimental brain tumors. Neurosurgical Focus 9, 1–6 (2000).
Lesniak, M. S., Tyler, B. M., Pardoll, D. M. & Brem, H. Gene therapy for experimental brain tumors using a xenogenic cell line engineered to secrete hIL-2. J. Neurooncol. 64, 155–160 (2003).
Hanes, J. et al. Controlled local delivery of interleukin-2 by biodegradable polymers protects animals from experimental brain tumors and liver tumors. Pharm. Res. 18, 899–906 (2001).
Sampath, P. et al. Paracrine immunotherapy with interleukin-2 and local chemotherapy is synergistic in the treatment of experimental brain tumors. Cancer Res. 59, 2107–2114 (1999).
Rhines, L. D. et al. Local immunotherapy with interleukin-2 delivered from biodegradable polymer microspheres combined with interstitial chemotherapy: a novel treatment for experimental malignant glioma. Neurosurgery 52, 872–879; discussion 879–880 (2003).
Acknowledgements
The research presented in this work has been supported in part by the National Cooperative Drug Discovery Group of the National Cancer Institute.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Under a licensing agreement between Guilford Pharmaceuticals and the Johns Hopkins University, H.B. is entitled to a share of royalty received by the University on sales of products described in this work. H.B. and the University own Guilford Pharmaceuticals stock, which is subject to certain restrictions under University policy. H.B. is also a paid consultant to Guilford Pharmaceuticals. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest polices.
Related links
Related links
DATABASES
Entrez Gene
O6-methylguanine-DNA methyltransferase
FURTHER INFORMATION
American Brain Tumor Association
MIT Program in Polymer Science and Technology
National Academy of Engineering
Encyclopedia of Life Sciences
Glossary
- SURGICAL DEBULKING
-
A surgical procedure in which part of the tumour is removed, as opposed to the entire tumour.
- BLOOD–BRAIN BARRIER
-
A state of physiological, metabolic and biochemical processes that distinguish the cerebral capillary endothelium from the endothelium of systemic organ systems. It is formed by tight junctions of cerebral capillary endothelial cells.
- TRANSCYTOSIS
-
The transport of material across an epithelium by uptake on one face into a coated vesicle, which then can be transported to the opposite face in another vesicle.
- PARENCHYMA
-
Tissue that constitutes the essential part of an organ, as contrasted with connective tissue and blood vessels.
- ARTERIOVENOUS SHUNTS
-
A passage by which blood is directly diverted from the arterial side to the venous side.
- VENOUS ANASTOMOSES
-
Communication between two or more veins forming a network of vessels.
- LIPOSOMES
-
Synthetic, uniform, bilayer lipid membrane vesicles formed by emulsification of cell membranes in dilute salt solutions. Liposomes are being developed as an approach for drug delivery in which toxic drugs are 'wrapped' inside a liposome and tagged with an organ-specific antibody.
- OMMAYA RESERVOIR
-
A device with a fluid reservoir implanted under the scalp with a catheter inside a ventricle. It allows for medication to be given directly to the cerebrospinal fluid and into the brain.
- HAZARD RATIO
-
A summary of the difference between two survival curves, representing the reduction in the risk of death on treatment compared with control, during the period of follow up.
- KARNOFSKY PERFORMANCE STATUS
-
A standard way of measuring the ability of cancer patients to perform ordinary tasks.
Rights and permissions
About this article
Cite this article
Lesniak, M., Brem, H. Targeted therapy for brain tumours. Nat Rev Drug Discov 3, 499–508 (2004). https://doi.org/10.1038/nrd1414
Issue Date:
DOI: https://doi.org/10.1038/nrd1414
This article is cited by
-
Ultrasound trapping and navigation of microrobots in the mouse brain vasculature
Nature Communications (2023)
-
Quantitative monitoring and modelling of retrodialysis drug delivery in a brain phantom
Scientific Reports (2023)
-
Conformable hierarchically engineered polymeric micromeshes enabling combinatorial therapies in brain tumours
Nature Nanotechnology (2021)
-
MicroRNA-138 suppresses glioblastoma proliferation through downregulation of CD44
Scientific Reports (2021)
-
Nanomedicine-based immunotherapy for central nervous system disorders
Acta Pharmacologica Sinica (2020)