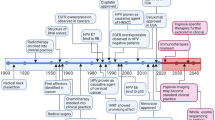
Abstract
Therapeutic management of locally advanced, recurrent and metastatic head and neck squamous cell carcinoma (HNSCC) is often limited by a rather unfavorable efficacy and toxicity ratio. Since the 1990s, targeted molecular therapy has been extensively investigated both as a single modality and in combination with cytotoxic treatments, such as radiotherapy or chemotherapy. EGFR is commonly over expressed in HNSCC and is an attractive molecular target. The EGFR signaling pathway is involved in a variety of cellular responses including cell growth and proliferation, and monoclonal antibodies and small-molecule inhibitors have been developed to inhibit EGFR pathways. Agents that target angiogenesis have also been tested in combination with EGFR inhibitors. A number of phase I/II and phase III studies have demonstrated that patients with high-risk HNSCC or those receiving palliative treatment for recurrent or metastatic disease benefit from the addition of EGFR inhibitors to chemotherapy and radiotherapy. This Review discusses the rationale for using targeted therapies based on inhibition of EGFR and angiogenesis, and describes the most recent preclinical and clinical evidence of the important role for targeted therapies in the management of head and neck cancers.
Key Points
-
On the basis of phase III data in patients with locally advanced HNSCC, coadministration of chemotherapy and radiotherapy yields higher rates of local control and survival than radiotherapy alone, but it is also considerably more toxic
-
Upregulation of EGFR signaling has an important role in the growth and metastasis of a wide range of tumors and is overexpressed in more than 90% of HNSCC
-
Monoclonal antibodies against EGFR and small-molecule tyrosine kinase inhibitors inhibit tumor growth, invasion and metastasis, DNA damage repair, and angiogenesis
-
In patients with locally advanced disease, cetuximab plus radiation significantly improves the median duration of locoregional control and overall survival, compared with radiation alone
-
The use of agents that target the outer and inner domains of EGFR is under investigation
-
Upregulation of VEGF levels by EGFR activation and cytotoxic agents justifies the use of molecular therapies that target both EGFR and VEGF
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Parkin, D. M., Bray, F., Ferlay, J. & Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 (2005).
Bernier, J. & Bentzen, S. M. Altered fractionation and combined radio-chemotherapy approaches: pioneering new opportunities in head and neck oncology. Eur. J. Cancer 39, 560–571 (2003).
Bentzen, S. M., Harari, P. M. & Bernier, J. Exploitable mechanisms for combining drugs with radiation: an overview of the concepts, achievements and future directions. Nat. Clin. Pract. Oncol. 4, 72–80 (2007).
Bernier, J. & Cooper, J. S. Chemoradiation after surgery for high-risk head and neck cancer patients: how strong is the evidence? Oncologist 10, 215–224 (2005).
Bentzen, S. M. & Trotti, A. Evaluation of early and late toxicities in chemoradiation trials. J. Clin. Oncol. 25, 4096–4103 (2007).
Bentzen, S. M. et al. Increasing toxicity in nonoperative head and neck cancer treatment: investigations and interventions. Int. J. Radiat. Oncol. Biol. Phys. 69 (Suppl. 2), S79–S82 (2007).
Clavel, M. et al. Randomized comparison of cisplatin, methotrexate, bleomycin and vincristine (CABO) versus cisplatin and 5-fluorouracil (CF) versus cisplatin (C) in recurrent or metastatic squamous cell carcinoma of the head and neck. A phase III study of the EORTC Head and Neck Cancer Cooperative Group. Ann. Oncol. 5, 521–526 (1994).
Forastiere, A. A. et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J. Clin. Oncol. 10, 1245–1251 (1992).
Jacobs, C. et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J. Clin. Oncol. 10, 257–263 (1992).
Colevas, A. D. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J. Clin. Oncol. 24, 2644–2652 (2006).
León, X. et al. A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. Clin. Oncol. (R. Coll. Radiol.) 17, 418–424 (2005).
Pfister, D. G. et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J. Clin. Oncol. 24, 1072–1078 (2006).
Kies, M. S. et al. Induction chemotherapy (CT) with weekly paclitaxel, carboplatin, and cetuximab for squamous cell carcinoma of the head and neck (HN) [Abstract]. ASCO Meeting Abstracts 24, 5520 (2006).
Bozec, A. et al. Dual inhibition of EGFR and VEGFR pathways in combination with irradiation: antitumour supra-additive effects on human head and neck cancer xenografts. Br. J. Cancer 97, 65–72 (2007).
Yarden, Y. & Sliwkowski, M. X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell. Biol. 2, 127–137 (2001).
Vivanco, I. & Sawyers, C.L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2, 489–501 (2002).
Choong, N. W. & Cohen, E. E. Epidermal growth factor receptor directed therapy in head and neck cancer. Crit. Rev. Oncol. Hematol. 57, 25–43 (2006).
Nyati, M. K., Morgan, M. A., Feng, F. Y. & Lawrence, T. S. Integration of EGFR inhibitors with radiochemotherapy. Nat. Rev. Cancer 6, 876–885 (2006).
Pectasides, E. et al. Evalutation of the prognostic significance of activated STAT3 expression levels in head and neck squamous cell cell carcinoma. ASCO Meeting Abstracts 26, 6015 (2008).
Pinkas-Kramarski, R. et al. Diversification of Neu differentiation factor and epidermal growth factor signalling by combinatorial receptor interactions. EMBO J. 15, 2452–2467 (1996).
Rubin Grandis, J., Chakraborty, A., Melhem, M. F., Zeng, Q. & Tweardy, D. J. Inhibition of epidermal growth factor receptor gene expression and function decreases proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. Oncogene 15, 409–416 (1997).
Shin, D. M., Ro, J. Y., Hong, W. K. & Hittelman, W. N. Dysregulation of epidermal growth factor receptor expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 54, 3153–3159 (1994).
Nozawa, H. et al. Small interfering RNA targeting epidermal growth factor receptor enhances chemosensitivity to cisplatin, 5-fluorouracil and docetaxel in head and neck squamous cell carcinoma. Cancer Sci. 97, 1115–1124 (2006).
Takes, R. P. et al. Differences in expression of oncogenes and tumor suppressor genes in different sites of head and neck squamous cell. Anticancer Res. 8, 4793–4800 (1998).
Maurizi, M. et al. Prognostic significance of epidermal growth factor receptor in laryngeal squamous cell carcinoma. Br. J. Cancer 74, 1253–1257 (1996).
Ang, K. K. et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 62, 7350–7356 (2002).
Garcia de Palazzo, I. E. et al. Expression of mutated epidermal growth factor receptor by non small cell lung cancer carcinomas. Cancer Res. 53, 3217–3220 (1993).
Moscatello, D. K. et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 55, 5536–5539 (1995).
Rodrigo, J. P., Ramos, S., Lazo, P. S., Alvarez, I. & Suárez, C. Amplification of ERBB oncogenes in squamous cell carcinomas of the head and neck. Eur. J. Cancer 32A, 2004–2010 (1996).
Todd, R. et al. TGF-alpha and EGFR receptors mRNAs in uhmann oral cancers. Carcinogenesis 10, 1553–1556 (1989).
Liu, W. et al. Interethnic difference in the allelic distribution of human epidermal growth factor receptor intron polymorphism. Clin. Cancer Res. 9, 1009–1012 (2003).
Mendelsohn, J. & Fan Z. Epidermal growth factor receptor family and chemosensitization. J. Natl Cancer Inst. 89, 341–343 (1997).
Ke, L. D., Adler-Stortlz, K., Clayman, G. L., Yung, A. W. & Chen, Z. Differential expression of epidermal growth factor receptor in human head and neck cancers. Head Neck 20, 320–327 (1998).
Chen, Z., Ke, L. D., Yuan, X. H. & Adler-Storthz, K. Correlation of cisplatin sensitivity with differential alteration of EGFR expression in head and neck cancer cells. Anticancer Res. 20, 899–902 (2000).
Dittmann, K. et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J. Biol. Chem. 280, 31182–31189 (2005).
Lammering, G. et al. Epidermal growth factor receptor as a genetic therapy target for carcinoma cell radiosensitization. J. Natl Cancer Inst. 93, 921–929 (2001).
Bowers, G. et al. The relative role of ErbB1–4 receptor tyrosine kinases in radiation signal transduction responses of human carcinoma cells. Oncogene 20, 1388–1397 (2001).
Li, S. et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7, 301–311 (2005).
Hadari, Y. R. et al. The IgG1 monoclonal antibody cetuximab induces degradation of the epidermal growth factor receptor [Abstract 234]. Presented at the ASCO Gastrointestinal Cancers Symposium, San Francisco, CA, January 23, 2004.
Choong, N. W. et al. Randomized phase II study of concomitant chemoradiotherapy with 5-fluorouracil-hydroxyurea (FHX) compared to FHX and bevacizumab (BFHX) in intermediate stage head and neck cancer (HNC) [Abstract]. ASCO Meeting Abstracts 25, 6034 (2007).
Huang, S. M., Li, J. & Harari, P. M. Molecular inhibition of angiogenesis and metastatic potential in human squamous cell carcinomas after epidermal growth factor receptor blockade. Mol. Cancer Ther. 1, 507–514 (2002).
Baselga J. The EGFR as a target for anticancer therapy—focus on cetuximab. Eur. J. Cancer 37 (Suppl. 4), S16–S22 (2001).
Ciardiello, F. et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin. Cancer Res. 7, 1459–1465 (2001).
Huang, S. M. & Harari, P. M. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin. Cancer Res. 6, 2166–2174 (2000).
Milas, L. et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin. Cancer Res. 6, 701–708 (2000).
Bonner, J. A. et al. Enhanced apoptosis with combination C225/radiation treatment serves as the impetus for clinical investigation in head and neck cancers. J. Clin. Oncol. 18 (Suppl. 21), S47–S53 (2000).
Nasu, S., Ang, K. K., Fan, Z. & Milas, L. C225 antiepidermal growth factor receptor antibody enhances tumor radiocurability. Int. J. Radiat. Oncol. Biol. Phys. 51, 474–477 (2001).
Saleh, M. N. et al. Combined modality therapy of A431 human epidermoid cancer using anti-EGFr antibody C225 and radiation. Cancer Biother. Radiopharm. 14, 451–463 (1999).
Huang, S. M., Bock, J. M. & Harari, P. M. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 59, 1935–1940 (1999).
Dittmann, K., Mayer, C. & Rodemann, H. P. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother. Oncol. 76, 157–161 (2005).
Krause, M. et al. Decreased repopulation as well as increased reoxygenation contribute to the improvement in local control after targeting of the EGFR by C225 during fractionated irradiation. Radiother. Oncol. 76, 162–167 (2005).
Nyati, M. K. et al. Radiosensitization by pan ErbB inhibitor CI-1033 in vitro and in vivo. Clin. Cancer Res. 10, 691–700 (2004).
Gorski, D. H. et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 59, 3374–3378 (1999).
Garcia-Barros, M. et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300, 1155–1159 (2003).
Peng, D. et al. Anti-epidermal growth factor receptor monoclonal antibody 225 upregulates p27KIP1 and induce G1 rest in prostatic cancer line DU145. Cancer Res. 56, 3666–3669 (1996).
Goldstein, N. I., Prewett, M., Zuklys, K., Rockwell, O. & Mendelsohn, J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin. Cancer Res. 1, 1311–1318 (1995).
Kang, X. et al. High affinity Fc receptor binding and potent inhibition of antibody-dependent cellular cytotoxicity (ADCC) in vitro by anti-epidermal growth factor receptor antibody cetuximab [Abstract]. ASCO Meeting Abstracts 25, 3041 (2007).
Kimura, H. et al. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 98, 1275–1280 (2007).
Rebucci, M. et al. Mitochondrial effects of combination cetuximab and ionizing radiation in head and neck squamous cell carcinoma cells [Abstract 391]. Eur. J. Cancer 5 (Suppl.) (2007).
Fan, Z., Baselga, J., Masui, H. & Mendelsohn, J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 53, 4637–4642 (1993).
Brown, D., Wang, R. & Russell, P. Antiepidermal growth factor receptor antibodies augment cytotoxicity of chemotherapeutic agents on squamous cell carcinoma cell lines. Otolaryngol. Head Neck Surg. 122, 75–83 (2000).
Baselga, J. et al. Phase ll multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J. Clin. Oncol. 23, 5568–5577 (2005).
Herbst, R. S. et al. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J. Clin. Oncol. 23, 5578–5587 (2005).
Vermorken, J. B. et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J. Clin. Oncol. 25, 2171–2177 (2007).
Burtness, B. Cetuximab and cisplatin for chemotherapy-refractory squamous cell cancer of the head and neck. J. Clin. Oncol. 23, 5440–5442 (2005).
Bourhis, J. et al. Phase I/II study of cetuximab in combination with cisplatin or carboplatin and fluorouracil in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. J. Clin. Oncol. 24, 2866–2872 (2006).
Vermorken, J. B. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 359, 1116–1127 (2008).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006).
Yang, X. A., Jia, X. C., Corvalan, J. R., Wang, P. & Davis, C. G. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody for cancer therapy. Crit. Rev. Oncol. Hematol. 38, 17–23 (2001).
Hoy, S. M. & Wagtsaff, A. J. Panitumumab in the treatment of metastatic colorectal cancer. Drugs 66, 2005–2014 (2006).
Cohenuram, M. & Saif, M. W. Panitumumab the first fully human monoclonal antibody: from the bench to the clinic. Anticancer Drugs 18, 7–15 (2007).
Yang, X. D. et al. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res. 59, 1236–1243 (1999).
Foon, K. A. et al. Preclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibody. Int. J. Radiat. Oncol. Biol. Phys. 58, 984–990 (2004).
Sartore-Bianchi, A. et al. Epidermal growth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J. Clin. Oncol. 25, 3238–3245 (2007).
Sasaki, H. et al. Nras and Kras mutation in Japanese lung cancer patients: Genotyping analysis using LightCycler. Oncol. Rep. 18, 623–628 (2007).
Ruiz-Godoy, R. L. M. et al. Mutational analysis of K-ras and Ras protein expression in larynx squamous cell carcinoma. J. Exp. Clin. Cancer Res. 25, 73–78 (2006).
Guyre, P. M., Graziano, R. F., Vance, B. A., Morganelli, P. M. & Fanger, M. W. Monoclonal antibodies that bind to distinct epitopes on Fc gamma RI are able to trigger receptor function. J. Immunol. 143, 1650–1655 (1989).
Crombet-Ramos, T., Rak, J., Perez, R. & Viloria-Petit, A. Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: a humanized anti-EGFR antibody. Int. J. Cancer 101, 567–575 (2002).
Shenoy, A. M. et al. BIOMAb EGFR TM (nimotuzumab/h-R3) in combination with standard care in squamous cell carcinoma of the head and neck. Presented at the 7th International Conference on head and neck cancer, San Francisco, CA, July 19–23 (2008).
Burger, A. M., Heiss, N. S. & Kreysch, H. The humanized monoclonal anti-EGFR antibody EMD72000 potently inhibits the growth of EGFR-expressing human tumor xenografts insensitive to chemotherapeutic drugs. Proc. Am. Assoc. Cancer Res. 44, 5719 (2003).
Modjtahedi, H. et al. Phase I trial and tumour localization of the anti-EGFR monoclonal antibody ICR62 in head and neck or lung cancer. Br. J. Cancer 73, 228–235 (1996).
[No authors listed] An open-Labeled trial with a dose-escalation part and a parallel group design investigating zalutumumab, an anti-EGF receptor antibody, in combination with chemo-radiation as first line treatment of patients with cancer of the head and neck. ClinicalTrials.gov [online November 2006] http://www.clinicaltrials.gov/ct2/show/NCT00401401?term=zalutumumab&rank=4.
Wakeling, A. E. et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signalling with potential for cancer therapy. Cancer Res. 62, 5749–5754 (2002).
Rojo, F. et al. Pharmacodynamic studies of tumor biopsy specimens from patients with advanced gastric carcinoma. J. Clin. Oncol. 24, 4309–4316 (2006).
Magne, N. et al. Influence of epidermal growth factor receptor (EGFR), p53 and intrinsic MAP kinase pathway status of tumour cells on the antiproliferative effect of Z1839 (Iressa). Br. J. Cancer 86, 1518–1523 (2002).
Eccles, S. A. et al. What are the determinants of response to EGFR signalling inhibitors in HNSCC? Eur. J. Cancer 5, (ECCO 14 abstract 49) (2007).
Servidei, T., Riccardi, A., Mozzetti, S., Ferlini, C. & Riccardi, R. Chemoresistant tumor cell lines display altered epidermal growth factor receptor and HER3 signaling and enhanced sensitivity to gefitinib. Int. J. Cancer, 123, 2939–2949 (2008).
Pollack, V. A. et al. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358 774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J. Pharmacol. Exp. Ther. 291, 739–748 (1999).
Bozec, A. et al. Combined effects of bevacizumab with erlotinib and irradiation: a preclinical study on a head and neck cancer orthotopic model. Br. J. Cancer 99, 93–99 (2008).
Xia, W., Liu, L. H., Ho, P. & Spector, N. L. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene 23, 646–653 (2004).
Spector, N. L. et al. Study of the biologic effects of lapatinib, a reversible inhibitor of erbB-1 and erbB-2 tyrosine kinase, on tumor growth and survival pathways in patients with advanced malignancies. J. Clin. Oncol. 23, 2502–2512 (2005).
Rusnak, D. W. et al. The effects of the novel, reversible epidermal growth factor receptor/erbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol. Cancer Ther. 1, 85–94 (2001).
Wood, E. R. et al. A unique structure for epidermal growth factor receptor bound to GW572016 (lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 64, 6662–6659 (2004).
Kane, M. A. et al. Phase II study of 250 mg gefitinib in advanced squamous cell carcinoma of the head and neck (SCCHN) [Abstract]. ASCO Meeting Abstracts 22, 5586 (2004).
Soulieres, D. et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J. Clin. Oncol. 22, 77–85 (2004).
Matar, P. et al. Combined anti-epidermal growth factor receptor (EGFR) treatment with a tyrosine kinase inhibitor gefitinib (ZD1839, Iressa) and a monoclonal antibody (IMC-C225): evidence of synergy. Proc. Am. Assoc. Cancer Res. 64, 800 (2004).
Huang, S., Armstrong, E. A., Benavente, S., Chinnaiyan, P. & Harari, P. M. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 64, 5355–5362 (2004).
Williams, K. J. et al. Combining radiotherapy with AZD2171, a potent inhibitor of vascular endothelial growth factor signaling: pathophysiologic effects and therapeutic benefit. Mol. Cancer Ther. 6, 599–606 (2007).
Perrotte, P. et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin. Cancer Res. 2, 257–265 (1999).
Woodburn, J. R. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol. Ther. 82, 241–250 (1999).
Hirata, A. et al. ZD1839 (Iressa) induces antiangiogenic effects through inhibition of epidermal growth factor receptor tyrosine kinase. Cancer Res. 62, 2554–2560 (2002).
Wedge, S. R. et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer signalling: Cancer Res. 65, 4389–4400 (2005).
Raben, D. et al. Antitumour activity of ZD6126, a novel vascular-targeting agent, is enhanced when combined with ZD1839, an epidermal growth factor receptor tyrosine kinase inhibitor, and potentiates the effects of radiation in a human non-small cell lung cancer xenograft model. Mol. Cancer Ther. 3, 977–983 (2004).
Yigitbasi, O. G. et al. Tumor cell and endothelial cell therapy of oral cancer by dual tyrosine kinase receptor blockade. Cancer Res. 64, 7977–7794 (2004).
Williams, K. J. et al. ZD6474, a potent inhibitor of vascular endothelial growth factor signaling, combined with radiotherapy: schedule-dependent enhancement of antitumor activity. Clin. Cancer Res. 10, 8587–8593 (2004).
Choe, M. S., Chen, Z., Klass, C. M., Zhang, X. & Shin, D. M. Enhancement of docetaxel-induced cytotoxicity by blocking epidermal growth factor receptor and cyclooxygenase-2 pathways in squamous cell carcinomas of the head and neck. Clin. Cancer Res. 13, 3015–3023 (2007).
Kubicek, J. G. et al. Phase I trial of bortezomib (VELCADE), cisplatin and radiotherapy for advanced head and neck cancer [Abstract]. ASCO Meeting Abstracts 26, 6028 (2008).
Bernier, J. et al. Consensus guidelines for the management of radiation dermatitis and co-existing acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann. Oncol. 19, 142–149 (2008).
Segaert, S. et al. The management of skin reactions in cancer patients receiving epidermal growth factor receptor targeted therapies. J. Dtsch Dermatol. Ges. 3, 599–606 (2005).
Segaert, S. & Van Cutsem, E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann. Oncol. 16, 1425–1433 (2005).
Lacouture, M. E. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat. Rev. Cancer 6, 803–812 (2006).
Jost, M., Kari, C. & Rodeck, U. The EGF receptor—an essential regulator of multiple epidermal functions. Eur. J. Dermatol. 10, 505–510 (2000).
Busam, K. J. et al. Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br. J. Dermatol. 144, 1169–1176 (2001).
Jacot, W. et al. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumors. Br. J. Dermatol. 151, 238–241 (2004).
Curran, D. et al. Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J. Clin. Oncol. 25, 2191–2197 (2007).
He, Y. et al. Inhibition of human squamous cell carcinoma growth in vivo by epidermal growth factor receptor antisense RNA transcribed from U6 promoter. J. Natl Cancer Inst. 90, 1080–1087 (1998).
Rubin Grandis, J. R. et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J. Natl Cancer Inst. 90, 824–828 (1998).
Schmidt, M. & Wels, W. Targeted inhibition of tumour cell growth by a bispecific single-chain toxin containing an antibody domain and TGF alpha. Br. J. Cancer 74, 853–862 (1996).
Mishra, G., Liu, T. F. & Frankel, A. E. Recombinant toxin DAB389RGF is cytotoxic to human pancreatic cancer cells. Expert Opin. Biol. Ther. 3, 1173–1180 (2003).
Masuelli, L. et al. Gene-specific inhibition of breast carcinoma in BALB-neuT mice by active immunization with rat Neu or human ErbB receptors. Int. J. Oncol. 30, 381–392 (2007).
Vokes, E. E. et al. A phase I dose escalation study of Ad GV.EGR.TNF.11D (TNFerade) with concurrent chemoradiotherapy in patients with recurrent head and neck cancer [Abstract]. ASCO Meeting Abstracts 26, 6067 (2008).
Fujikawa, T. et al. Cimetidine inhibits epidermal growth factor-induced cell signaling. J. Gastroenterol. Hepatol. 22, 436–443 (2007).
Argiris, A. E. et al. Phase II trial of neoadjuvant docetaxel (T), cisplatin (P), and cetuximab followed by concurrent radiation (X), P, and E in locally advanced head and neck cancer (HNC) [Abstract]. ASCO Meeting Abstracts 26, 6002 (2008).
Tishler, R. B. et al. Cetuximab added to docetaxel, cisplatin, 5-fluorouracil induction chemotherapy (C-TPF) in patients with new diagnosed locally advanced head and neck cancer: A phase I study [Abstract]. ASCO Meeting Abstracts 26, 6001 (2008).
Bonnin, N. et al. Efficacy of neoadjuvant TPF (nTPF : docetaxel, T ; cisplatin: P; 5FU) in non selected patients with head and neck cancer and subsequent radiotherapy combined with chemotherapy and cetuximab [Abstract]. ASCO Meeting Abstracts 26, 6074 (2008).
Langer, C. J. et al. Preliminary analysis of ECOG 3303: concurrent radiation, cisplatin, and cetuximab in unresectable, locally advanced squamous cell carcinoma of the head and neck [Abstract]. ASCO Meeting Abstracts 26, 6006 (2008).
[No authors listed] A phase II randomized trial of surgery followed by chemoradiotherapy plus cetuximab for advanced squamous cell carcinoma of the head and neck. ClincialTrials.gov [online April 2004] http://www.clinicaltrials.gov/ct2/show/NCT00084318?term=cetuximab +%2B+radiotherapy+%2B+docetaxel+%2B+cisplatin&rank=4.
Wirth, L. J. et al. Phase I study of panitumumab + chemoradiotherapy for head and neck cancer [Abstract]. ASCO Meeting Abstracts 26, 6007 (2008).
[No authors listed.] A randomised, double-blind, placebo-controlled, multicentre, phase III study of post-operative adjuvant lapatinib or placebo and concurrent chemoradiotherapy followed by maintenance lapatinib or placebo monotherapy in high-risk subjects with resected squamous cell carcinoma of the head and neck (SCCHN). ClinicalTrials.gov [online December 2006] http://www.clinicaltrials.gov/ct2/show/ NCT00424255?term=lapatinib+AND+postoperative&rank=1.
Arias de la Vega, F. et al. Erlotinib and chemoradiation in patients with surgically resected locally advanced squamous head and neck cancer: a GICOR phase I study [Abstract]. ASCO Meeting Abstracts 26, 6068 (2008).
[No authors listed.] CARISSA Trial—multicenter randomized phase II trial comparing post-operative radiotherapy and cisplatin alone or in combination with the EGFR inhibitor ZD 1839 (Iressa) in upper aerodigestive tract carcinomas. ClinicalTrials.gov [online September 2005] http://www.clinicaltrials.gov/ct2/show/NCT00169221?term=carissa&rank=1.
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non small cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Mellinghoff, I. K. et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024 (2005).
Lee, J. W. et al. Somatic mutations of EGFR gene in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 11, 2879–2882 (2005).
Cappuzzo, F. et al. Epidermal growth factor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J. Natl Cancer Inst. 97, 643–655 (2005).
Amador, M. L. et al. An epidermal growth factor receptor intron 1 polymorphism mediates response to epidermal growth factor receptor inhibitors. Cancer Res. 64, 9139–9143 (2004).
Hirsch, F. R. et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity in gefitinib in patients with bronchoalveolar carcinoma subtypes: a Southwest Oncology Group Study. J. Clin. Oncol. 23, 6838–6845 (2005).
Takano, T. et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small cell lung cancer. J. Clin. Oncol. 23, 6829–6837 (2005).
Johnson, B. E. & Jänne, P. A. Selecting patients for epidermal growth factor receptor inhibitor treatment: a FISH story or a tale of mutations? J. Clin. Oncol. 23, 6813–6816 (2005).
Kumar, B. et al. EGFR, p16, HPV titer, bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J. Clin. Oncol. 26, 3128–3137 (2008).
Milas, L. et al. Importance of maintenance therapy in C225-induced enhancement of tumor control by fractionated radiation. Int. J. Radiat. Oncol. Biol. Phys. 67, 568–572 (2007).
Robert, F. et al. Phase I study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J. Clin. Oncol. 19, 3234–3243 (2001).
Su, Y. B. et al. Concurrent cetuximab, cisplatin, and radiotherapy (RT) for loco-regionally advanced squamous cell carcinoma of the head and neck (SCCHN): updated results of a novel combined modality paradigm [Abstract]. ASCO Meeting Abstracts 23, 5529 (2005).
Merlano, M. C. et al. Cetuximab (C-mab) and chemo-radiation (CT-RT) for loco-regional advanced squamous cell carcinoma of the head and neck (HNC): A phase II study [Abstract]. ASCO Meeting Abstracts 25, 6043 (2007).
Kuhnt, T. et al. Concomitant hyperfractionated accelerated radiotherapy (HART) with cisplatin and concurrent cetuximab for locoregionally advanced squamous cell head and neck cancer: a phase I dose escalation trial [Abstract]. ASCO Meeting Abstracts 26, 6029 (2008).
Savvides, P. et al. Phase II study of bevacizumab with docetaxel and radiation in locally advanced head and neck squamous cell cancer. Presented at the 7th International Conference on head and neck cancer, San Francisco, CA, July 19–23 (2008).
Herchenhorn, D. et al. Phase II study of erlotinib combined with cisplatin and radiotherapy for locally advanced squamous cell carcinoma of the head and neck (SCCHN) [Abstract]. ASCO Meeting Abstracts 25, 6033 (2007).
Cohen, E. E. et al. Integration of gefitinib (G.), in to a concurrent chemoradiation (CRT) regimen followed by G. adjuvant therapy in patients with locally advanced head and neck cancer (HNC)—a phase II trial [Abstract]. ASCO Meeting Abstracts 23, 5506 (2005).
Chen, C. et al. Phase I trial of gefitinib in combination with radiation or chemoradiation for patients with locally advanced squamous cell head and neck cancer. J. Clin. Oncol. 25, 4880–4886 (2007).
Rueda, A. et al. Gefitinib plus concomitant boost accelerated radiation (AFX-CB) and concurrent weekly cisplatin for locally advanced unresectable squamous cell head and neck carcinomas (SCCHN): A phase II study [Abstract]. ASCO Meeting Abstracts 25, 6031 (2007).
Adelstein, D. J. et al. Concurrent chemoradiotherapy and gefitinib for locoregionally advanced head and neck squamous cell cancer. Presented at the 7th International Conference on head and neck cancer, San Francisco, CA, July 19–23 (2008).
Doss, H. H. et al. Induction chemotherapy + gefitinib followed by concurrent chemotherapy/radiation therapy/gefitinib for patients (pts) with locally advanced squamous cell carcinoma of the head and neck: A phase I/II trial of the Minnie Pearl Cancer Research Network [Abstract]. ASCO Meeting Abstracts 24, 5543 (2006).
Ahmed, S. M. et al. Updated results of a phase II trial integrating gefitinib (G.) into concurrent chemoradiation (CRT) followed by G. adjuvant therapy for locally advanced head and neck cancer (HNC) [Abstract]. ASCO Meeting Abstracts 25, 6028 (2007).
El-Hariry, I., Harrington, K., Bourhis, J. & Holford, C. A phase I, open-label, dose escalation study (EGF 100262) of lapatinib plus chemoradiation in patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN) [Abstract 91]. Radiother. Oncol. 82, S30 (2007).
Chan, A. T. C. et al. A phase II study of cetuximab (C225) in combination with carboplatin in patients(pts) with recurrent or metastatic nasopharyngeal carcinoma (NPC) who failed to a platinum-based chemotherapy [Abstract]. ASCO Meeting Abstracts 22, 2000 (2003).
Hitt, R. et al. Phase II study of combination cetuximab and weekly paclitaxel in patients with metastatic/recurrent squamous cell carcinoma of head and neck (SCCHN): Spanish Head and Neck Cancer Group (TTCC) [Abstract]. ASCO Meeting Abstracts 25, 6012 (2007).
Knoedeler, M. et al. Phase II trial to evaluate efficacy and toxicity of cetuximab plus docetaxel in platinum pretreated patients with recurrent and/or metastatic head and neck cancer [Abstract]. ASCO Meeting Abstracts 26, 6066 (2008).
Kies, M. S. et al. Cetuximab and bevacizumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an interim analysis. ASCO Meeting Abstracts 26, 6072 (2008).
Kirby, A. M. et al. Gefitinib (ZD1839, Iressa) as palliative treatment in recurrent and metastatic head and neck cancer. Br. J. Cancer 94, 631–636 (2006).
Chua, D. T., Sham, J. & Au, G. A phase II trial of gefitinib in recurrent and metastatic nasopharyngeal carcinoma pretreated with platinum-based chemotherapy [Abstract]. ASCO Meeting Abstracts 25, 6042 (2007).
Cohen, E. E. et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J. Clin. Oncol. 21, 1980–1987 (2003).
Wheeler, R. H. et al. Clinical and molecular phase II study of gefitinib in patients with recurrent squamous cell cancer of the head and neck [Abstract]. ASCO Meeting Abstracts 25, 5531 (2005).
Wirth, L. J. et al. Phase I study of gefitinib plus celecoxib in patients with metastatic and/or locally recurrent squamous cell carcinoma of the head and neck (SCCHN) [Abstract]. ASCO Meeting Abstracts 22, 5540 (2004).
Vokes, E. E. et al. A phase I study of erlotinib and bevacizumab for recurrent or metastatic squamous cell carcinoma of the head and neck (HNC) [Abstract]. ASCO Meeting Abstracts 23, 5504 (2005).
Kim, E. S. et al. Phase II study of combination cisplatin, docetaxel and erlotinib in patients with metastatic/recurrent head and neck squamous cell carcinoma (HNSCC) [Abstract]. ASCO Meeting Abstracts 25, 5546 (2005).
Mauer, A. M. et al. Phase I study of epidermal growth factor receptor (RGFR) inhibitor, erlotinib, and vascular endothelial growth factor monoclonal antibody, bevacizumab, in recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN) [Abstract]. ASCO Meeting Abstracts 22, 5539 (2004).
Abidoye, O. O. et al. Phase II study of GW572016 in squamous cell carcinoma of the head and neck [Abstract]. ASCO Meeting Abstracts 24, 5568 (2006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Jacques Bernier is a consultant for Amgen, GlaxoSmithKline, Merck Serono and Sanofi–Aventis, and is also on the speakers bureau for Amgen, Merck Serono and Sanofi–Aventis. Jan B. Vermorken is a consultant and is on the speakers bureau for Amgen, Merck Serono and Sanofi–Aventis. Søren M. Bentzen declared no competing interests.
Rights and permissions
About this article
Cite this article
Bernier, J., Bentzen, S. & Vermorken, J. Molecular therapy in head and neck oncology. Nat Rev Clin Oncol 6, 266–277 (2009). https://doi.org/10.1038/nrclinonc.2009.40
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.40