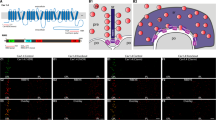
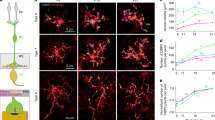
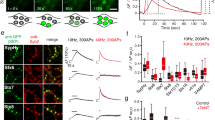
Abstract
Synaptic ribbons with a halo of synaptic vesicles are seen at the active zones of sensory neurons that release transmitter tonically. Thus, ribbons are assumed to be a prerequisite for sustained exocytosis. By applying total internal reflection fluorescence microscopy to goldfish retinal bipolar cell terminals, we visualized Ca2+ entry sites, ribbons, and vesicle fusion events. Here we show that the main Ca2+ entry sites were located at ribbons, and that activation of the Ca2+ current induced immediate and delayed vesicle fusion events at ribbon-associated and ribbon-free 'hot spots', respectively. The activation of protein kinase C (PKC) specifically potentiated vesicle fusion at ribbon-free sites. Electron microscopy showed that PKC activation selectively increased the number of docked vesicles at ribbon-free sites, which faced neuronal processes with the postsynaptic density. Retinal bipolar cells have both ribbon-associated and ribbon-free active zones in their terminals and might send functionally distinct signals through ribbon-associated and ribbon-free synapses to postsynaptic neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
von Gersdorff, H., Vardi, E., Matthews, G. & Sterling, P. Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron 16, 1221–1227 (1996).
Lenzi, D., Crum, J., Ellisman, M.H. & Roberts, W.M. Depolarization redistributes synaptic membrane and creates a gradient of vesicles on the synaptic body at a ribbon synapse. Neuron 36, 649–659 (2002).
tom Dieck, S. et al. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J. Cell Biol. 168, 825–836 (2005).
Lenzi, D. & von Gersdorff, H. Structure suggests function: the case for synaptic ribbons as exocytotic nanomachines. Bioessays 23, 831–840 (2001).
Zhai, R.G. & Bellen, H.J. The architecture of the active zone in the presynaptic nerve terminal. Physiology (Bethesda) 19, 262–270 (2004).
Dowling, J.E. The Retina: an Approachable Part of the Brain (Harvard Univ. Press, Cambridge, Massachusetts, 1987).
Zenisek, D., Davila, V., Wan, L. & Almers, W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J. Neurosci. 23, 2538–2548 (2003).
Zenisek, D., Horst, N.K., Merrifield, C., Sterling, P. & Matthews, G. Visualizing synaptic ribbons in the living cell. J. Neurosci. 24, 9752–9759 (2004).
Zenisek, D., Steyer, J.A. & Almers, W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature 406, 849–854 (2000).
Zenisek, D., Steyer, J.A., Feldman, M.E. & Almers, W. A membrane marker leaves synaptic vesicles in milliseconds after exocytosis in retinal bipolar cells. Neuron 35, 1085–1097 (2002).
Llobet, A., Cooke, A. & Lagnado, L. Exocytosis at the ribbon synapse of retinal bipolar cells studied in patches of presynaptic membrane. J. Neurosci. 23, 2706–2714 (2003).
Mennerick, S. & Matthews, G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron 17, 1241–1249 (1996).
Sakaba, T., Tachibana, M., Matsui, K. & Minami, N. Two components of transmitter release in retinal bipolar cells: exocytosis and mobilization of synaptic vesicles. Neurosci. Res. 27, 357–370 (1997).
von Gersdorff, H., Sakaba, T., Berglund, K. & Tachibana, M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron 21, 1177–1188 (1998).
Suzuki, S. & Kaneko, A. Identification of bipolar cell subtypes by protein kinase C-like immunoreactivity in the goldfish retina. Vis. Neurosci. 5, 223–230 (1990).
Osborne, N.N., Wood, J. & Groome, N. The occurrence of three calcium-independent protein kinase C subspecies (delta, epsilon and zeta) in retina of different species. Brain Res. 637, 156–162 (1994).
Minami, N., Berglund, K., Sakaba, T., Kohmoto, H. & Tachibana, M. Potentiation of transmitter release by protein kinase C in goldfish retinal bipolar cells. J. Physiol. (Lond.) 512, 219–225 (1998).
Berglund, K., Midorikawa, M. & Tachibana, M. Increase in the pool size of releasable synaptic vesicles by the activation of protein kinase C in goldfish retinal bipolar cells. J. Neurosci. 22, 4776–4785 (2002).
von Gersdorff, H. Synaptic ribbons: versatile signal transducers. Neuron 29, 7–10 (2001).
Neher, E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20, 389–399 (1998).
Tachibana, M., Okada, T., Arimura, T., Kobayashi, K. & Piccolino, M. Dihydropyridine-sensitive calcium current mediates neurotransmitter release from bipolar cells of the goldfish retina. J. Neurosci. 13, 2898–2909 (1993).
Steyer, J.A. & Almers, W. Tracking single secretory granules in live chromaffin cells by evanescent-field fluorescence microscopy. Biophys. J. 76, 2262–2271 (1999).
Becherer, U., Moser, T., Stuhmer, W. & Oheim, M. Calcium regulates exocytosis at the level of single vesicles. Nat. Neurosci. 6, 846–853 (2003).
Diggle, P.J. Statistical Analysis of Spatial Point Patterns (Arnold, London, 2003).
Allen, M.B. Structure, physiology, and biochemistry of the chryophyceae. Annu. Rev. Microbiol. 23, 29–46 (1969).
Hull, C., Studholme, K., Yazulla, S. & von Gersdorff, H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J. Neurophysiol. 96, 2025–2033 (2006).
Raviola, E. & Raviola, G. Structure of the synaptic membranes in the inner plexiform layer of the retina: a freeze-fracture study in monkeys and rabbits. J. Comp. Neurol. 209, 233–248 (1982).
Roberts, W.M., Jacobs, R.A. & Hudspeth, A.J. The hair cell as a presynaptic terminal. Ann. NY Acad. Sci. 635, 221–233 (1991).
von Gersdorff, H. & Matthews, G. Depletion and replenishment of vesicle pools at a ribbon-type synaptic terminal. J. Neurosci. 17, 1919–1927 (1997).
Heidelberger, R., Heinemann, C., Neher, E. & Matthews, G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature 371, 513–515 (1994).
Heidelberger, R. Adenosine triphosphate and the late steps in calcium-dependent exocytosis at a ribbon synapse. J. Gen. Physiol. 111, 225–241 (1998).
Feigenspan, A. & Bormann, J. Modulation of GABAC receptors in rat retinal bipolar cells by protein kinase C. J. Physiol. (Lond.) 481, 325–330 (1994).
Gillette, M.A. & Dacheux, R.F. Protein kinase modulation of GABAA currents in rabbit retinal rod bipolar cells. J. Neurophysiol. 76, 3070–3086 (1996).
Job, C. & Lagnado, L. Calcium and protein kinase C regulate the actin cytoskeleton in the synaptic terminal of retinal bipolar cells. J. Cell Biol. 143, 1661–1672 (1998).
Khimich, D. et al. Hair cell synaptic ribbons are essential for synchronous auditory signaling. Nature 434, 889–894 (2005).
Dick, O. et al. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron 37, 775–786 (2003).
Ball, S.L. et al. Role of the β2 subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest. Ophthalmol. Vis. Sci. 43, 1595–1603 (2002).
Tachibana, M. & Okada, T. Release of endogenous excitatory amino acids from ON-type bipolar cells isolated from the goldfish retina. J. Neurosci. 11, 2199–2208 (1991).
Suzuki, S., Tachibana, M. & Kaneko, A. Effects of glycine and GABA on isolated bipolar cells of the mouse retina. J. Physiol. (Lond.) 421, 645–662 (1990).
Acknowledgements
We thank T. Asaka for participation in early experiments and S. Tokimura for excellent technical assistance. This work was supported by JSPS Grants-in-Aid for Scientific Research (18300132) and Grant-in-Aid for Scientific Research on Priority Areas from MEXT (12053212, 18019012) to M.T.
Author information
Authors and Affiliations
Contributions
M.M. conducted the main body of the physiological experiments, Y.T. carried out the electron microscopic studies, K.B. contributed the data analysis of the electron microscopy, M.I. carried out the immunohistochemistry, and M.T. wrote the manuscript and supervised the project.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Table 1 (PDF 128 kb)
Supplementary Video 1
Synaptic ribbons and Ca2+ entry sites. Visualization by fluorescein-conjugated CtBP-biding peptide and Fluo-5F. A goldfish Mb1 bipolar cell terminal under a TIRF microscope with 488-nm illumination. Before depolarization, five ribbons are observed. Upon depolarization, Ca2+ emerged at ribbons. Picture size is 10.24 × 10.24 μm (40 nm pixel−1). (MOV 123 kb)
Supplementary Video 2
Fusion of FM-labeled vesicles in a goldfish Mb1 bipolar cell terminal under a TIRF microscope. Images were acquired for 3.5 s every 36.4 ms. Picture size is 10.24 × 10.24 μm (40 nm pixel−1). (MOV 257 kb)
Supplementary Video 3
Fusion of 'Resident'. The vesicle intensity was already high before fusion. Picture size is 1.24 × 1.24 μm (40 nm pixel−1). Averaged image from ten fusion events. (MOV 57 kb)
Supplementary Video 4
Fusion of 'Newcomer'. The vesicle intensity was increased abruptly from background before fusion. Picture size is 1.24 × 1.24 μm (40 nm pixel−1). Averaged image from five fusion events. (MOV 71 kb)
Rights and permissions
About this article
Cite this article
Midorikawa, M., Tsukamoto, Y., Berglund, K. et al. Different roles of ribbon-associated and ribbon-free active zones in retinal bipolar cells. Nat Neurosci 10, 1268–1276 (2007). https://doi.org/10.1038/nn1963
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1963
This article is cited by
-
Transmission at rod and cone ribbon synapses in the retina
Pflügers Archiv - European Journal of Physiology (2021)
-
Dynamic assembly of ribbon synapses and circuit maintenance in a vertebrate sensory system
Nature Communications (2019)
-
In Vivo Ribbon Mobility and Turnover of Ribeye at Zebrafish Hair Cell Synapses
Scientific Reports (2017)
-
Live-cell imaging of receptors around postsynaptic membranes
Nature Protocols (2014)
-
Retinal bipolar cells: elementary building blocks of vision
Nature Reviews Neuroscience (2014)