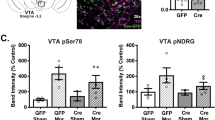
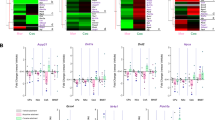
Abstract
The transcription factor ΔFosB is induced in the nucleus accumbens (NAc) and dorsal striatum by the repeated administration of drugs of abuse. Here, we investigated the role of ΔFosB in the NAc in behavioral responses to opiates. We achieved overexpression of ΔFosB by using a bitransgenic mouse line that inducibly expresses the protein in the NAc and dorsal striatum and by using viral-mediated gene transfer to specifically express the protein in the NAc. ΔFosB overexpression in the NAc increased the sensitivity of the mice to the rewarding effects of morphine and led to exacerbated physical dependence, but also reduced their sensitivity to the analgesic effects of morphine and led to faster development of analgesic tolerance. The opioid peptide dynorphin seemed to be one target through which ΔFosB produced this behavioral phenotype. Together, these experiments demonstrated that ΔFosB in the NAc, partly through the repression of dynorphin expression, mediates several major features of opiate addiction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Robinson, T.E. & Berridge, K.C. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95 (suppl. 2), S91–S117 (2000).
Wise, R.A. Drug-activation of brain reward pathways. Drug Alcohol Depend. 51, 13–22 (1998).
Koob, G.F., Sanna, P.P. & Bloom, F.E. Neuroscience of addiction. Neuron 21, 467–476 (1998).
Everitt, B.J. & Wolf, M.E. Psychomotor stimulant addiction: a neural systems perspective. J. Neurosci. 22, 3312–3320 (2002).
Hyman, S.E. & Malenka, R.C. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat. Rev. Neurosci. 2, 695–703 (2001).
Nestler, E.J. Molecular basis of long-term underlying addiction. Nat. Rev. Neurosci. 2, 119–128 (2001).
Kalivas, P.W. Glutamate systems in cocaine addiction. Curr. Opin. Pharmacol. 4, 23–29 (2004).
Yao, W.D. et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron 41, 625–638 (2004).
Asanuma, M. & Cadet, J.L. Methamphetamine-induced increase in striatal NF-kappaB DNA-binding activity is attenuated in superoxide dismutase transgenic mice. Brain Res. Mol. Brain Res. 60, 305–309 (1998).
Carlezon, W.A. et al. Regulation of cocaine reward by CREB. Science 282, 2272–2275 (1998).
O'Donovan, K.J., Tourtellotte, W.G., Millbrandt, J. & Baraban, J.M. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 22, 167–173 (1999).
Barrot, M. et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc. Natl. Acad. Sci. USA 99, 11435–11440 (2002).
Marinelli, M. & Piazza, P.V. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur. J. Neurosci. 16, 387–394 (2002).
McClung, C.A. & Nestler, E.J. Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nat. Neurosci. 6, 1208–1215 (2003).
Hope, B.T. et al. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 13, 1235–1244 (1994).
Moratalla, R., Elibol, B., Vallejo, M. & Graybiel, A.M. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron 17, 147–156 (1996).
Nye, H.E. & Nestler, E.J. Induction of chronic fos-related antigens in rat brain by chronic morphine administration. Mol. Pharmacol. 49, 636–645 (1996).
Hiroi, N. et al. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine's psychomotor and rewarding effects. Proc. Natl. Acad. Sci. USA 94, 10397–10402 (1997).
Pich, E.M., Pagliusi, S.R., Tessari, M., Talabot-Ayer, D., Hooft van Huijsduijnen, R. & Chiamulera, C. Common neural substrates for the addictive properties of nicotine and cocaine. Science 275, 83–86 (1997).
Ehrlich, M.E., Sommer, J., Canas, E. & Unterwald, E.M. Periadolescent mice show enhanced DeltaFosB upregulation in response to cocaine and amphetamine. J. Neurosci. 22, 9155–9159 (2002).
Werme, M. et al. ΔFosB regulates wheel running. J. Neurosci. 22, 8133–8138 (2002).
Chen, J., Kelz, M.B., Hope, B.T., Nakabeppu, Y. & Nestler, E.J. Chronic FRAs: Stable variants of ΔFosB induced in brain by chronic treatments. J. Neurosci. 17, 4933–4941 (1997).
Kelz, M.B. et al. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature 401, 272–276 (1999).
Colby, C.R., Whisler, K., Steffen, C., Nestler, E.J. & Self, D.W. ΔFosB enhances incentive for cocaine. J. Neurosci. 23, 2488–2493 (2003).
Peakman, M.C. et al. Inducible, brain region-specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res. 970, 73–86 (2003).
Funada, M., Suzuki, T., Narita, M., Misawa, M. & Nagase, H. Blockade of morphine reward through the activation of kappa-opioid receptors in mice. Neuropharmacology 32, 1315–1323 (1993).
Wang, X.M., Zhou, Y., Spangler, R., Ho, A., Han, J.S. & Kreek, M.J. Acute intermittent morphine increases preprodynorphin and kappa opioid receptor mRNA levels in the rat brain. Brain Res. Mol. Brain Res. 66, 184–187 (1999).
Shippenberg, T.S., Chefer, V.I., Zapata, A. & Heidbreder, C.A. Modulation of the behavioural and neurochemical effects of psychostimulants by kapa-opioid receptor systems. Ann. N.Y. Acad. Sci. 937, 50–73 (2001).
Akil, H. & Watson, S.J. Cloning of kappa opioid receptors: functional significance and future directions. Prog. Brain Res. 100, 81–86 (1994).
Svingos, A.L., Colago, E.E. & Pickel, V.M. Cellular sites for dynorphin activation of kappa opioid receptor in the rat nucleus accumbens shell. J. Neurosci. 19, 1804–1812 (1999).
Matthes, H.W. et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383, 819–823 (1996).
Frenois, F., Cador, M., Caille, S., Stinus, L. & Le Moine, C. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur. J. Neurosci. 16, 1377–1389 (2002).
Maldonado, R., Stinus, L., Gold, L.H. & Koob, G.F. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J. Pharmacol. Exp. Ther. 261, 669–677 (1992).
Harris, G.C. & Aston-Jones, G. Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature 371, 155–157 (1994).
Harris, G.C. & Aston-Jones, G. Altered motivation and learning following opiate withdrawal: evidence for prolonged dysregulation of reward processing. Neuropsychopharmacology 28, 865–871 (2003).
Shippenberg, T.S., Heidbreder, C. & Lefevour, A. Sensitization to the conditioned rewarding effects of morphine: pharmacology and temporal characteristics. Eur. J. Pharmacol. 299, 33–39 (1996).
Willis, W.D. & Westlund, K.N. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 14, 2–31 (1997).
Inturrisi, C.E. Clinical pharmacology of opioids for pain. Clin. J. Pain 18 (suppl.), S3–13, 2002.
Brog, J.S., Salyapongse, A., Deutch, A.Y. & Zahm, D.S. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol. 338, 255–278 (1993).
Gear, R.W. & Levine, J.D. Antinociception produced by an ascending spino-supraspinal pathway. J. Neurosci. 15, 3154–3161 (1995).
Altier, N. & Stewart, J. The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 65, 2269–2287 (1999).
Schmidt, B.L . et al. Altered nucleus accumbens circuitry mediates pain induced antinociception in morphine tolerant rats. J. Neurosci. 15, 6773–6780 (2002).
Suzuki, T., Narita, M., Takahashi, Y., Misawa, M. & Nagase, H. Effects of nor-binaltorphimine on the development of analgesic tolerance and physiological dependence on morphine. Eur. J. Pharmacol. 213, 91–97 (1992).
Cole, R.L., Konradi, C., Douglass, J. & Hyman, S.E. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron 14, 813–823 (1995).
Shaw-Lutchman, T.Z. et al. Regional and cellular mapping of cAMP response element-mediated transcription during naltrexone-precipitated morphine withdrawal. J. Neurosci. 22, 3663–3672 (2002).
Yukhananov, R.Yu., Zhai, Q.Z., Persson, S., Post, C. & Nyberg, F. Chronic administration of morphine decreases level of dynorphin A in the rat nucleus accumbens. Neuropharmacology 32, 703–709 (1993).
Nylander, I., Vlaskova, M. & Terenius, L. The effects of morphine treatment and morphine withdrawal on the dynorphin and enkephalin systems in Sprague-Dawley rats. Psychopharmacology (Berl.) 118, 391–400 (1995).
Chefer, V.I. et al. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J. Neurosci. 25, 5029–5037 (2005).
Shippenberg, T.S., LeFevour, A. & Thompson, A.C. Sensitization to the conditioned rewarding effects of morphine and cocaine: differential effects of the kappa-opioid receptor agonist U69593. Eur. J. Pharmacol. 345, 27–34 (1998).
Zachariou, V. et al. Essential role for RGS9 in opiate action. Proc. Natl. Acad. Sci. USA 100, 13656–13661 (2003).
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse and National Institute of Mental Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Tables
Opioid Receptor Levels and Opiate Withdrawal Behaviors in Inducible Bitransgenic Mice (PDF 97 kb)
Rights and permissions
About this article
Cite this article
Zachariou, V., Bolanos, C., Selley, D. et al. An essential role for ΔFosB in the nucleus accumbens in morphine action. Nat Neurosci 9, 205–211 (2006). https://doi.org/10.1038/nn1636
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1636
This article is cited by
-
Methamphetamine-induced region-specific transcriptomic and epigenetic changes in the brain of male rats
Communications Biology (2023)
-
Key transcription factors mediating cocaine-induced plasticity in the nucleus accumbens
Molecular Psychiatry (2022)
-
Kappa opioid receptors mediate an initial aversive component of paclitaxel-induced neuropathy
Psychopharmacology (2020)
-
Influence of pharmacological and epigenetic factors to suppress neurotrophic factors and enhance neural plasticity in stress and mood disorders
Cognitive Neurodynamics (2019)
-
Transgenerational transmission of hedonic behaviors and metabolic phenotypes induced by maternal overnutrition
Translational Psychiatry (2018)