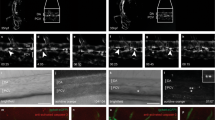
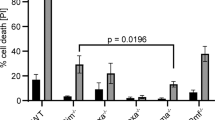
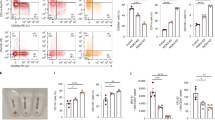
Abstract
Bag1 is a cochaperone for the heat-shock protein Hsp70 that interacts with C-Raf, B-Raf, Akt, Bcl-2, steroid hormone receptors and other proteins. Here we use targeted gene disruption in mice to show that Bag1 has an essential role in the survival of differentiating neurons and hematopoietic cells. Cells of the fetal liver and developing nervous system in Bag1−/− mice underwent massive apoptosis. Lack of Bag1 did not disturb the primary function of Akt or Raf, as phosphorylation of the forkhead transcription factor FKHR and activation of extracellular signal–regulated kinase (Erk)-1/2 were not affected. However, the defect was associated with the disturbance of a tripartite complex formed by Akt, B-Raf and Bag1, in addition to the absence of Bad phosphorylation at Ser136. We also observed reduced expression of members of the inhibitor of apoptosis (IAP) family. Our data show that Bag1 is a physiological mediator of extracellular survival signals linked to the cellular mechanisms that prevent apoptosis in hematopoietic and neuronal progenitor cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Takayama, S. et al. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 16, 4887–4896 (1997).
Bimston, D. et al. BAG-1, a negative regulator of hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J. 17, 6871–6878 (1998).
Stuart, J.K. et al. Characterization of interactions between the anti-apoptotic protein BAG- 1 and Hsc70 molecular chaperones. J. Biol. Chem. 273, 22506–22514 (1998).
Brehmer, D. et al. Tuning of chaperone activity of Hsp70 proteins by modulation of nucleotide exchange. Nat. Struct. Biol. 8, 427–432 (2001).
Takayama, S. & Reed, J.C. Molecular chaperone targeting and regulation by BAG family proteins. Nat. Cell Biol. 3, E237–E241 (2001).
Takayama, S. et al. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell 80, 279–284 (1995).
Wang, H.G. & Reed, J.C. Bc1–2, Raf-1 and mitochondrial regulation of apoptosis. Biofactors 8, 13–16 (1998).
Kermer, P. et al. BAG1 over-expression in brain protects against stroke. Brain Pathol. 13, 495–506 (2003).
Eversole-Cire, P. et al. Synergistic effect of Bcl-2 and BAG-1 on the prevention of photoreceptor cell death. Invest. Ophthalmol. Vis. Sci. 41, 1953–1961 (2000).
Lee, M.Y. et al. Reactive astrocytes express bis, a bcl-2-binding protein, after transient forebrain ischemia. Exp. Neurol. 175, 338–346 (2002).
Jiang, Y., Woronicz, J.D., Liu, W. & Goeddel, D.V. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science 283, 543–546 (1999).
Miki, K. & Eddy, E.M. Tumor necrosis factor receptor 1 is an ATPase regulated by silencer of death domain. Mol. Cell. Biol. 22, 2536–2543 (2002).
Thress, K., Song, J., Morimoto, R.I. & Kornbluth, S. Reversible inhibition of Hsp70 chaperone function by Scythe and Reaper. EMBO J. 20, 1033–1041 (2001).
Thress, K., Henzel, W., Shillinglaw, W. & Kornbluth, S. Scythe: a novel reaper-binding apoptotic regulator. EMBO J. 17, 6135–6143 (1998).
Wang, H.G., Takayama, S., Rapp, U.R. & Reed, J.C. Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc. Natl. Acad. Sci. USA 93, 7063–7068 (1996).
Wang, H.G., Rapp, U.R. & Reed, J.C. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell 87, 629–638 (1996).
Datta, S.R. et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–241 (1997).
Datta, S.R. et al. 14–3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol. Cell 6, 41–51 (2000).
Datta, S.R. et al. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev. Cell 3, 631–643 (2002).
Wiese, S. et al. Specific function of B-Raf in mediating survival of embryonic motoneurons and sensory neurons. Nat. Neurosci. 4, 137–142 (2001).
Froesch, B.A., Takayama, S. & Reed, J.C. BAG-1L protein enhances androgen receptor function. J. Biol. Chem. 273, 11660–11666 (1998).
Hohfeld, J. & Jentsch, S. GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 16, 6209–6216 (1997).
Schmidt, U. et al. Essential role of the unusual DNA-binding motif of BAG-1 for inhibition of the glucocorticoid receptor. J. Biol. Chem. 278, 4926–4931 (2003).
Yenari, M.A., Giffard, R.G., Sapolsky, R.M. & Steinberg, G.K. The neuroprotective potential of heat shock protein 70 (HSP70). Mol. Med. Today 5, 525–531 (1999).
Coldwell, M.J. et al. The p36 isoform of BAG-1 is translated by internal ribosome entry following heat shock. Oncogene 20, 4095–4100 (2001).
Crocoll, A., Blum, M. & Cato, A.C. Isoform-specific expression of BAG-1 in mouse development. Mech. Dev. 91, 355–359 (2000).
Lo, A.C., Houenou, L.J. & Oppenheim, R.W. Apoptosis in the nervous system: morphological features, methods, pathology, and prevention. Arch. Histol. Cytol. 58, 139–149 (1995).
Kermer, P. et al. Bag1 is a regulator and marker of neuronal differentiation. Cell Death Differ. 9, 405–413 (2002).
Tran, J. et al. Marked induction of the IAP family antiapoptotic proteins survivin and XIAP by VEGF in vascular endothelial cells. Biochem. Biophys. Res. Commun. 264, 781–788 (1999).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
von Gise, A. et al. Apoptosis suppression by Raf-1 and MEK1 requires MEK- and phosphatidylinositol 3-kinase-dependent signals. Mol. Cell. Biol. 21, 2324–2336 (2001).
Jablonka, S., Wiese, S. & Sendtner, M. Axonal defects in mouse models of motoneuron disease. J. Neurobiol. 58, 272–286 (2004).
Guan, K.L. et al. Negative regulation of the serine/threonine kinase B-Raf by Akt. J. Biol. Chem. 275, 27354–27359 (2000).
Michaelidis, T.M. et al. Inactivation of the bcl-2 gene results in progressive degeneration of motoneurons, sensory and sypathetic neurons during early postnatal development. Neuron 17, 75–89 (1996).
Gross, A., McDonnell, J.M. & Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13, 1899–1911 (1999).
Laprise, P. et al. Merosin (laminin-2/4)-driven survival signaling: complex modulations of Bcl-2 homologs. J. Cell. Biochem. 89, 1115–1125 (2003).
Wiese, S. et al. The anti-apoptotic protein ITA is essential for NGF-mediated survival of embryonic chick neurons. Nat. Neurosci. 2, 978–983 (1999).
Wang, C.Y., Mayo, M.W., Korneluk, R.G., Goeddel, D.V. & Baldwin, A.S.J. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281, 1680–1683 (1998).
Beg, A.A., Sha, W.C., Bronson, R.T., Ghosh, S. & Baltimore, D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 376, 167–170 (1995).
Deshmukh, M. & Johnson, E.M., Jr . Programmed cell death in neurons: focus on the pathway of nerve growth factor deprivation-induced death of sympathetic neurons. Mol. Pharmacol. 51, 897–906 (1997).
Acknowledgements
We thank M. Pfister, J. Marcano and K. Kalus for excellent technical assistance; R. McKay (US National Institutes of Health) for providing the nestin-specific antibody; T. Jessell for providing the islet-1/2 hybridoma (39.4D5) through the Developmental Studies Hybridoma Bank; and R. Timpl for the kind donation of laminin. This work was supported by the Deutsche Forschungsgemeinschaft (SE 697/3-3, SFB487, TP C4 and SFB581 TP B4 and B17), the US National Institutes of Health (CA67385) and the Hermann and Lilly Schilling Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Activated caspase 3 immunoreactivity is increased in E10.5 and E11.5 bag-1−/− embryonic forebrain. (PDF 7732 kb)
Supplementary Fig. 2
Enhanced apoptosis of differentiating neurons from bag-1−/− Pax6-positive neural stem cells. (PDF 3237 kb)
Supplementary Fig. 3
Knockdown of Bag-1 by RNAi results in loss of Bag-1 immunohistochemistry. (PDF 775 kb)
Supplementary Fig. 4
Enhanced apoptosis of differentiating neurons in the forebrain of bag-1−/− mice. (PDF 8622 kb)
Supplementary Fig. 5
Model for the interaction of a complex formed by Bag-1, Hsp70, B-Raf and Akt and its substrate Bad in wild-type mice and disturbance of this process in bag-1−/− mice. (PDF 157 kb)
Supplementary Table 1
Number of apoptotic cells in spinal cord and brain sections of bag-1+/+ and bag-1−/− mice. (PDF 36 kb)
Rights and permissions
About this article
Cite this article
Götz, R., Wiese, S., Takayama, S. et al. Bag1 is essential for differentiation and survival of hematopoietic and neuronal cells. Nat Neurosci 8, 1169–1178 (2005). https://doi.org/10.1038/nn1524
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1524
This article is cited by
-
TNF-alpha-induced microglia activation requires miR-342: impact on NF-kB signaling and neurotoxicity
Cell Death & Disease (2020)
-
Protein expression pattern of the molecular chaperone Mdg1/ERdj4 during embryonic development
Histochemistry and Cell Biology (2020)
-
Bag-1 stimulates Bad phosphorylation through activation of Akt and Raf kinases to mediate cell survival in breast cancer
BMC Cancer (2019)
-
Over-expression of BAG-1 in head and neck squamous cell carcinomas (HNSCC) is associated with cisplatin-resistance
Journal of Translational Medicine (2017)
-
BAG-1L Protects SH-SY5Y Neuroblastoma Cells Against Hypoxia/Re-oxygenation Through Up-Regulating HSP70 and Activating PI3K/AKT Signaling Pathway
Neurochemical Research (2017)