Abstract
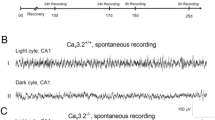
In humans, mutations in the KCNQ2 or KCNQ3 potassium-channel genes are associated with an inherited epilepsy syndrome. We have studied the contribution of KCNQ/M-channels to the control of neuronal excitability by using transgenic mice that conditionally express dominant-negative KCNQ2 subunits in brain. We show that suppression of the neuronal M current in mice is associated with spontaneous seizures, behavioral hyperactivity and morphological changes in the hippocampus. Restriction of transgene expression to defined developmental periods revealed that M-channel activity is critical to the development of normal hippocampal morphology during the first postnatal weeks. Suppression of the M current after this critical period resulted in mice with signs of increased neuronal excitability and deficits in hippocampus-dependent spatial memory. M-current-deficient hippocampal CA1 pyramidal neurons showed increased excitability, reduced spike-frequency adaptation, attenuated medium afterhyperpolarization and reduced intrinsic subthreshold theta resonance. M channels are thus critical determinants of cellular and neuronal network excitability, postnatal brain development and cognitive performance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Brown, D.A. & Adams, P.R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 283, 673–676 (1980).
Brown, D.A. M-currents: an update. Trends Neurosci. 11, 294–299 (1988).
Halliwell, J.V. & Adams, P.R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 250, 71–92 (1982).
Storm, J.F. Potassium currents in hippocampal pyramidal cells. Prog. Brain Res. 83, 161–187 (1990).
Jentsch, T.J. Neuronal KCNQ potassium channels: physiology and role in disease. Nat. Rev. Neurosci. 1, 21–30 (2000).
Ryan, S.G. et al. Benign familial neonatal convulsions: evidence for clinical and genetic heterogeneity. Ann. Neurol. 29, 469–473 (1991).
Dedek, K., Fusco, L., Teloy, N. & Steinlein, O.K. Neonatal convulsions and epileptic encephalopathy in an Italian family with a missense mutation in the fifth transmembrane region of KCNQ2. Epilepsy Res. 54, 21–27 (2003).
Borgatti, R. et al. A novel mutation in KCNQ2 associated with BFNC, drug resistant epilepsy, and mental retardation. Neurology 63, 57–65 (2004).
Dedek, K. et al. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proc. Natl. Acad. Sci. USA 98, 12272–12277 (2001).
Marrion, N.V. Control of M-current. Annu. Rev. Physiol. 59, 483–504 (1997).
Wang, H.S. et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282, 1890–1893 (1998).
Selyanko, A.A. et al. Inhibition of KCNQ1–4 potassium channels expressed in mammalian cells via M1 muscarinic acetylcholine receptors. J. Physiol. (Lond.) 522, 349–355 (2000).
Hadley, J.K. et al. Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium. J. Neurosci. 23, 5012–5019 (2003).
Cooper, E.C. et al. Colocalization and coassembly of two human brain M-type potassium channel subunits that are mutated in epilepsy. Proc. Natl. Acad. Sci. USA 97, 4914–4919 (2000).
Shah, M.M., Mistry, M., Marsh, S.J., Brown, D.A. & Delmas, P. Molecular correlates of the M-current in cultured rat hippocampal neurons. J. Physiol. (Lond.) 544, 29–37 (2002).
Cooper, E.C., Harrington, E., Jan, Y.N. & Jan, L.Y. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J. Neurosci. 21, 9529–9540 (2001).
Devaux, J.J., Kleopa, K.A., Cooper, E.C. & Scherer, S.S. KCNQ2 is a nodal K+ channel. J. Neurosci. 24, 1236–1244 (2004).
Storm, J.F. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J. Physiol. (Lond.) 409, 171–190 (1989).
Madison, D.V. & Nicoll, R.A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J. Physiol. (Lond.) 354, 319–331 (1984).
Malenka, R.C., Madison, D.V., Andrade, R. & Nicoll, R.A. Phorbol esters mimic some cholinergic actions in hippocampal pyramidal neurons. J. Neurosci. 6, 475–480 (1986).
Watanabe, H. et al. Disruption of the epilepsy KCNQ2 gene results in neural hyperexcitability. J. Neurochem. 75, 28–33 (2000).
Yang, Y. et al. Spontaneous deletion of epilepsy gene orthologs in a mutant mouse with a low electroconvulsive threshold. Hum. Mol. Genet. 12, 975–984 (2003).
Gossen, M. & Bujard, H. Tight control of gene expression in mammalian cells by tetracycline- responsive promoters. Proc. Natl. Acad. Sci. USA 89, 5547–5551 (1992).
Tremblay, P. et al. Doxycycline control of prion protein transgene expression modulates prion disease in mice. Proc. Natl. Acad. Sci. USA 95, 12580–12585 (1998).
Schroeder, B.C., Kubisch, C., Stein, V. & Jentsch, T.J. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature 396, 687–690 (1998).
Hu, H., Vervaeke, K. & Storm, J.F. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J. Physiol. (Lond.) 545, 783–805 (2002).
Nitecka, L. et al. Maturation of kainic acid seizure-brain damage syndrome in the rat. II. Histopathological sequelae. Neuroscience 13, 1073–1094 (1984).
Mello, L.E. et al. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia 34, 985–995 (1993).
Holmes, G.L. & Ben-Ari, Y. Seizures in the developing brain: perhaps not so benign after all. Neuron 21, 1231–1234 (1998).
Stocker, M., Krause, M. & Pedarzani, P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc. Natl. Acad. Sci. USA 96, 4662–4667 (1999).
Hutcheon, B. & Yarom, Y. Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 23, 216–222 (2000).
Pike, F.G. et al. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J. Physiol. (Lond.) 529, 205–213 (2000).
Leung, L.S. & Yu, H.W. Theta-frequency resonance in hippocampal CA1 neurons in vitro demonstrated by sinusoidal current injection. J. Neurophysiol. 79, 1592–1596 (1998).
Hutcheon, B., Miura, R.M. & Puil, E. Subthreshold membrane resonance in neocortical neurons. J. Neurophysiol. 76, 683–697 (1996).
Morris, R.G., Garrud, P., Rawlins, J.N. & O'Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683 (1982).
Buzsaki, G. Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002).
Sah, P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 19, 150–154 (1996).
Schroeder, B.C., Hechenberger, M., Weinreich, F., Kubisch, C. & Jentsch, T.J. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M- type currents. J. Biol. Chem. 275, 24089–24095 (2000).
Vergara, C., Latorre, R., Marrion, N.V. & Adelman, J.P. Calcium-activated potassium channels. Curr. Opin. Neurobiol. 8, 321–329 (1998).
Bond, C.T. et al. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J. Neurosci. 24, 5301–5306 (2004).
Williamson, A. & Alger, B.E. Characterization of an early afterhyperpolarization after a brief train of action potentials in rat hippocampal neurons in vitro. J. Neurophysiol. 63, 72–81 (1990).
Raghavachari, S. et al. Gating of human theta oscillations by a working memory task. J. Neurosci. 21, 3175–3183 (2001).
Kahana, M.J., Seelig, D. & Madsen, J.R. Theta returns. Curr. Opin. Neurobiol. 11, 739–744 (2001).
Sarnthein, J., Petsche, H., Rappelsberger, P., Shaw, G.L. & von Stein, A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl. Acad. Sci. USA 95, 7092–7096 (1998).
Blümcke, I., Thom, M. & Wiestler, O.D. Ammon's horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol. 12, 199–211 (2002).
Khazipov, R. et al. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur. J. Neurosci. 19, 590–600 (2004).
Ben-Ari, Y. Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 3, 728–739 (2002).
Lechner, S.G., Mayer, M. & Boehm, S. Activation of M1 muscarinic receptors triggers transmitter release from rat sympathetic neurons through an inhibition of M-type K+ channels. J. Physiol. (Lond.) 553, 789–802 (2003).
Martire, M. et al. M channels containing KCNQ2 subunits modulate norepinephrine, aspartate, and GABA release from hippocampal nerve terminals. J. Neurosci. 24, 592–597 (2004).
Noebels, J.L. The biology of epilepsy genes. Annu. Rev. Neurosci. 26, 599–625 (2003).
Acknowledgements
We thank S.B. Prusiner, UCSF, for providing the tg(Prnp-tTA) mouse line and H. Voss for animal caretaking and execution of doxycycline application protocols. We also thank F. Morellini for help with behavioral testing and statistical analysis, S. Fehr and I. Hermans-Borgmeyer for help with in situ hybridizations, F. Kutschera for developing the cage activity recorder, and S. Schillemeit and C. Petterson Oksvold for technical assistance. This study was supported by grants of the German Federal Ministry of Education and Research, as a part of the National Genome Research Network (NGFN), to D.I. and O.P. Research by H.H. and J.F.S. was supported by the European Commission (Contract No. QLG3-1999-00827), and by the Norwegian Research Council (NFR) Medicine & Health (MH) and Norwegian Centre of Excellence (SFF) programs.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
In situ hybridization analyses in hippocampus. In situ hybridization (ISH) experiments using a 35S-labeled hKCNQ2-specific antisense probe in male control (a), mutant (b), mutant on dox (c), WDW mutant (d), and female mutant mice (e). The smaller images illustrate magnifications of the CA1 area marked by a dotted rectangle. In order to visualize cell nuclei in ISH experiments, sections were stained with hemalaun. Scale bar: 500 µm (JPG 65 kb)
Supplementary Fig. 2
Identification of a critical period for phenotype development using the Tet-Off system. Mutant animals were administered doxycycline during different periods of pre- and postnatal development, thus giving rise to dox-water (DW), water-dox-water (WDW), or water-dox (WD) mutants. During the period from conception until weaning, doxycycline was added to the drinking water of the mothers. After weaning doxycycline was added to the drinking water of the offspring. Periods of doxycycline application (transgene expression off) are marked in red, periods of pure water application (transgene expression on) are marked in blue. The morphological hippocampal phenotype of the animals was assessed at 12 to 16 weeks of age. The dotted line marks the critical period during which transgene expression was associated with markedly altered hippocampal morphology and severe behavioral abnormalities. # Three females out of more than 100 animals occasionally showed hyperactivity and circling behavior. * The phenotype of DW mice treated with doxycycline until P0 was variable. This was probably due to residual doxycycline in the brains of the neonates and to a delayed increase in transgene expression. (GIF 10 kb)
Supplementary Fig. 3
Comparison of resonance behavior of CA1 pyramidal neurons after blocking INaP with TTX. Representative voltage responses to ZAP current injection into CA1 pyramidal cells from mutants on dox (a, n = 7 cells from 4 mice) and mutant mice (b, n = 10 cells from 5 mice). The cells were depolarized to –48 mV by current injection. Note that mutant cells showed little resonance behavior. (c) and (d) Effect of XE991 application (10 µM) on the resonance behavior of the same cells as shown in (a) (XE991 was tested in 5 of the 7 mutant on dox cells) and (b) (XE991 was tested on 8 of the 10 mutant cells). Note that XE911 suppressed the resonance in mutant on dox cells (c), but it had little effect on the resonance in mutant cells (d). (e and (f) Plots of impedance magnitude as a function of input frequency before (black) and after (blue) application of XE911 (10 µM) from the same cells shown in (a)-(d). (g) and (h) Summary plot of the resonance frequency and Q factors from mutant and mutant on dox cells before and after application of XE991 in the presence of TTX. (GIF 23 kb)
Supplementary Fig. 4
Comparison of resonance behavior of CA1 pyramidal neurons at hyperpolarized membrane potentials (H-resonance). Resonance at hyperpolarized membrane potentials was tested by injecting an oscillating current with linearly increasing frequency (ZAP current) into the cell, starting from a holding level of 7-9 mV. Typical membrane potential response to a ZAP current injection in cells from mutants on dox (a, n=5 cells from 2 mice) and mutants (b, n=7 cells from 2 mice). (c, d) Impedence plotted as a function of input frequency calculted from the data shown in (a) and (b). Mutant on dox cells (c, e) and mutant cells (d, e) showed a resonance peak between 2 and 3 Hz. (f) As indicated by the Q factors, the strength of the observed resonance was unchanged in mutant on dox and in mutant cells. (GIF 11 kb)
Supplementary Fig. 5
Comparison of I/V plots in CA1 pyramidal neurons from mutant on dox and mutant mice. Current-voltage (I/V) plots from >mutant on dox cells (filled circles, >n = 6 cells from 2 mice) and mutant cells (open circles, n = 8 cells from 3 mice). The cells were kept in a medium with 3.5 mM [K+] at their natural resting membrane potential (–72.31 ± 2.32 mV for mutant on dox cells, and –74.72 ± 1.54 mV for mutant cells), and 400-ms-long current pulses of different intensities were injected into the cells. The membrane potential responses to the current pulses (measured at the end of each pulse) were plotted against the amplitude of the current pulses (which were all subthreshold for spike generation). Note that current pulses larger than 0.15 nA evoked significantly less depolarization in mutant on dox than in mutant cells (* - P < 0.05). (GIF 6 kb)
Rights and permissions
About this article
Cite this article
Peters, H., Hu, H., Pongs, O. et al. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci 8, 51–60 (2005). https://doi.org/10.1038/nn1375
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1375
This article is cited by
-
A small-molecule activation mechanism that directly opens the KCNQ2 channel
Nature Chemical Biology (2024)
-
Nine patients with KCNQ2-related neonatal seizures and functional studies of two missense variants
Scientific Reports (2023)
-
Genetic Knockout of TRPM2 Increases Neuronal Excitability of Hippocampal Neurons by Inhibiting Kv7 Channel in Epilepsy
Molecular Neurobiology (2022)
-
Place fields of single spikes in hippocampus involve Kcnq3 channel-dependent entrainment of complex spike bursts
Nature Communications (2021)
-
Epileptic channelopathies caused by neuronal Kv7 (KCNQ) channel dysfunction
Pflügers Archiv - European Journal of Physiology (2020)