Abstract
The E693Q mutation in the amyloid beta precursor protein (APP) leads to cerebral amyloid angiopathy (CAA), with recurrent cerebral hemorrhagic strokes and dementia. In contrast to Alzheimer disease (AD), the brains of those affected by hereditary cerebral hemorrhage with amyloidosis–Dutch type (HCHWA-D) show few parenchymal amyloid plaques. We found that neuronal overexpression of human E693Q APP in mice (APPDutch mice) caused extensive CAA, smooth muscle cell degeneration, hemorrhages and neuroinflammation. In contrast, overexpression of human wild-type APP (APPwt mice) resulted in predominantly parenchymal amyloidosis, similar to that seen in AD. In APPDutch mice and HCHWA-D human brain, the ratio of the amyloid-β40 peptide (Aβ40) to Aβ42 was significantly higher than that seen in APPwt mice or AD human brain. Genetically shifting the ratio of AβDutch40/AβDutch42 toward AβDutch42 by crossing APPDutch mice with transgenic mice producing mutated presenilin-1 redistributed the amyloid pathology from the vasculature to the parenchyma. The understanding that different Aβ species can drive amyloid pathology in different cerebral compartments has implications for current anti-amyloid therapeutic strategies. This HCHWA-D mouse model is the first to develop robust CAA in the absence of parenchymal amyloid, highlighting the key role of neuronally produced Aβ to vascular amyloid pathology and emphasizing the differing roles of Aβ40 and Aβ42 in vascular and parenchymal amyloid pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Haass, C. & Steiner, H. Protofibrils, the unifying toxic molecule of neurodegenerative disorders? Nat. Neurosci. 4, 859–860 (2001).
Hardy, J. Amyloid, the presenilins and Alzheimer's disease. Trends. Neurosci. 20, 154–159 (1997).
Selkoe, D.J. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature 399, A23–31 (1999).
Levy, E. et al. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 248, 1124–1126 (1990).
Wattendorff, A.R., Bots, G.T., Went, L.N. & Endtz, L.J. Familial cerebral amyloid angiopathy presenting as recurrent cerebral haemorrhage. J. Neurol. Sci. 55, 121–135 (1982).
Bornebroek, M., Haan, J., Maat-Schieman, M.L., Van Duinen, S.G. & Roos, R.A. Hereditary cerebral hemorrhage with amyloidosis-Dutch type (HCHWA-D). I. A review of clinical, radiologic and genetic aspects. Brain. Pathol. 6, 111–114 (1996).
Maat-Schieman, M.L., van Duinen, S.G., Bornebroek, M., Haan, J. & Roos, R.A. Hereditary cerebral hemorrhage with amyloidosis-Dutch type (HCHWA-D). II. A review of histopathological aspects. Brain. Pathol. 6, 115–120 (1996).
Vinters, H.V. Cerebral amyloid angiopathy. A critical review. Stroke 18, 311–324 (1987).
Greenberg, S.M. Cerebral amyloid angiopathy: prospects for clinical diagnosis and treatment. Neurology 51, 690–694 (1998).
Fraser, P.E. et al. Fibril formation by primate, rodent, and Dutch-hemorrhagic analogues of Alzheimer amyloid β-protein. Biochemistry 31, 10716–10723 (1992).
De Jonghe, C. et al. Flemish and Dutch mutations in amyloid β precursor protein have different effects on amyloid β secretion. Neurobiol. Dis. 5, 281–286 (1998).
Watson, D.J., Selkoe, D.J. & Teplow, D.B. Effects of the amyloid precursor protein Glu693ÆGln 'Dutch' mutation on the production and stability of amyloid β-protein. Biochem. J. 340 (Pt 3), 703–709 (1999).
Monro, O.R. et al. Substitution at codon 22 reduces clearance of Alzheimer's amyloid-β peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol. Aging 23, 405–412 (2002).
Van Nostrand, W.E., Melchor, J.P. & Ruffini, L. Pathologic amyloid β-protein cell surface fibril assembly on cultured human cerebrovascular smooth muscle cells. J. Neurochem. 70, 216–223 (1998).
Klafki, H.W., Wiltfang, J. & Staufenbiel, M. Electrophoretic separation of βA4 peptides (1–40) and (1–42). Anal. Biochem. 237, 24–29 (1996).
Citron, M. et al. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nat. Med. 3, 67–72 (1997).
De Jonghe, C. et al. Evidence that Aβ42 plasma levels in presenilin-1 mutation carriers do not allow for prediction of their clinical phenotype. Neurobiol. Dis. 6, 280–287 (1999).
Castano, E.M. et al. The length of amyloid-β in hereditary cerebral hemorrhage with amyloidosis, Dutch type. Implications for the role of amyloid-β 1–42 in Alzheimer's disease. J. Biol. Chem. 271, 32185–32191 (1996).
Prelli, F. et al. Expression of a normal and variant Alzheimer's β-protein gene in amyloid of hereditary cerebral hemorrhage, Dutch type: DNA and protein diagnostic assays. Biochem. Biophys. Res. Commun. 170, 301–307 (1990).
Jankowsky, J.L. et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Hum. Mol. Genet. 13, 159–170 (2004).
Johnson-Wood, K. et al. Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 94, 1550–1555 (1997).
Richards, J.G. et al. PS2APP transgenic mice, coexpressing hPS2mut and hAPPswe, show age-related cognitive deficits associated with discrete brain amyloid deposition and inflammation. J. Neurosci. 23, 8989–9003 (2003).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
Nilsberth, C. et al. The 'Arctic' APP mutation (E693G) causes Alzheimer's disease by enhanced Aβ protofibril formation. Nat. Neurosci. 4, 887–893 (2001).
Tsubuki, S., Takaki, Y. & Saido, T.C. Dutch, Flemish, Italian, and Arctic mutations of APP and resistance of Aβ to physiologically relevant proteolytic degradation. Lancet 361, 1957–1958 (2003).
Morelli, L. et al. Differential degradation of amyloid β genetic variants associated with hereditary dementia or stroke by insulin-degrading enzyme. J. Biol. Chem. 278, 23221–23226 (2003).
Borchelt, D.R. et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19, 939–945 (1997).
Holcomb, L. et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 4, 97–100 (1998).
Natte, R. et al. Ultrastructural evidence of early non-fibrillar Aβ42 in the capillary basement membrane of patients with hereditary cerebral hemorrhage with amyloidosis, Dutch type. Acta Neuropathol. (Berl.) 98, 577–582 (1999).
Meyer-Luehmann, M. et al. Extracellular amyloid formation and associated pathology in neural grafts. Nat. Neurosci. 6, 370–377 (2003).
Shibata, M. et al. Clearance of Alzheimer's amyloid-β(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 106, 1489–1499 (2000).
Weller, R.O. et al. Cerebral amyloid angiopathy: amyloid β accumulates in putative interstitial fluid drainage pathways in Alzheimer's disease. Am. J. Pathol. 153, 725–733 (1998).
Pfeifer, M. et al. Cerebral hemorrhage after passive anti-Aβ immunotherapy. Science 298, 1379 (2002).
Das, P., Murphy, M.P., Younkin, L.H., Younkin, S.G. & Golde, T.E. Reduced effectiveness of Aβ1–42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol. Aging 22, 721–727 (2001).
Nicoll, J.A. et al. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat. Med. 9, 448–452 (2003).
Koller, M.F. et al. Active immunization of mice with an Aβ-Hsp70 vaccine. Neurodegenerative Dis. 1, 20–28 (2004).
Ferrer, I., Boada Rovira, M., Sánchez Guerra, M.L., Rey, M.J. & Costa-Jussá, F. Neuropathology and pathogenesis of encephalitis following amyloid-β immunization in Alzheimer's disease. Brain Pathol. 14, 11–20 (2004).
Natte, R. et al. Dementia in hereditary cerebral hemorrhage with amyloidosis-Dutch type is associated with cerebral amyloid angiopathy but is independent of plaques and neurofibrillary tangles. Ann. Neurol. 50, 765–772 (2001).
Greenberg, S.M. Cerebral amyloid angiopathy and dementia: two amyloids are worse than one. Neurology 58, 1587–1588 (2002).
Christie, R., Yamada, M., Moskowitz, M. & Hyman, B. Structural and functional disruption of vascular smooth muscle cells in a transgenic mouse model of amyloid angiopathy. Am. J. Pathol. 158, 1065–1071 (2001).
Winkler, D.T. et al. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J. Neurosci. 21, 1619–1627 (2001).
Calhoun, M.E. et al. Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc. Natl. Acad. Sci. USA 96, 14088–14093 (1999).
Van Dorpe, J. et al. Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the london mutant of human APP in neurons. Am. J. Pathol. 157, 1283–1298 (2000).
Sturchler-Pierrat, C. et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA 94, 13287–13292 (1997).
Bodendorf, U. et al. Expression of human β-secretase in the mouse brain increases the steady-state level of β-amyloid. J. Neurochem. 80, 799–806 (2002).
Mehta, P.D. et al. Plasma and cerebrospinal fluid levels of amyloid β proteins 1–40 and 1–42 in Alzheimer disease. Arch. Neurol. 57, 100–105 (2000).
Ohsawa, K., Imai, Y., Kanazawa, H., Sasaki, Y. & Kohsaka, S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J. Cell. Sci. 113 (Pt 17), 3073–3084 (2000).
Janus, C. et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature 408, 979–982 (2000).
Rozmahel, R. et al. Normal brain development in PS1 hypomorphic mice with markedly reduced γ-secretase cleavage of βAPP. Neurobiol. Aging 23, 187–194 (2002).
Maat-Schieman, M.L., Yamaguchi, H., van Duinen, S.G., Natte, R. & Roos, R.A. Age-related plaque morphology and C-terminal heterogeneity of amyloid β in Dutch-type hereditary cerebral hemorrhage with amyloidosis. Acta Neuropathol. (Berl.) 99, 409–419 (2000).
Acknowledgements
We would like to thank H. Widmer for experimental help; L. Walker, J. Ghiso and T. Saido for comments on this manuscript; and R. Nixon for support of the ELISA measurements. This work was supported by grants to M.J. from the Fritz Thyssen Foundation (Cologne, Germany), the EU under the sixth framework programme (priority: life sciences and health, LSHM-CT-2003-503330) and the Swiss National Science Foundation. This work was also supported by grants to P.M.M. from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
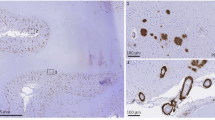
Intraneuronal Aβ in APPDutch and APPwt transgenic mice. NT12-immunostaining for Aβ (brown) in hippocampal CA1 neurons reveals intracellular human Aβ in a 29 month-old APPDutch (a) and a 24 month-old APPwt mouse (b), while no Aβ is detected in a 24 month-old non-transgenic control littermate (c). Bars are 20 μm (a–c). (JPG 56 kb)
Supplementary Table 1
Absolute Aβ levels in brains of pre-depositing and depositing transgenic mice, and HCHWA-D and AD patients. (PDF 19 kb)
Rights and permissions
About this article
Cite this article
Herzig, M., Winkler, D., Burgermeister, P. et al. Aβ is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci 7, 954–960 (2004). https://doi.org/10.1038/nn1302
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1302
This article is cited by
-
Aberrant palmitoylation caused by a ZDHHC21 mutation contributes to pathophysiology of Alzheimer’s disease
BMC Medicine (2023)
-
Critical thinking of Alzheimer’s transgenic mouse model: current research and future perspective
Science China Life Sciences (2023)
-
Disrupted Maturation of Prefrontal Layer 5 Neuronal Circuits in an Alzheimer’s Mouse Model of Amyloid Deposition
Neuroscience Bulletin (2023)
-
Early impairment of cortical circuit plasticity and connectivity in the 5XFAD Alzheimer’s disease mouse model
Translational Psychiatry (2022)
-
Aktuelle Befunde zur Koinzidenz von zerebraler Amyloidangiopathie und Alzheimer-Erkrankung
Der Nervenarzt (2022)