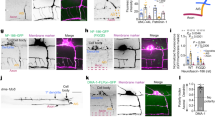
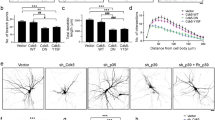
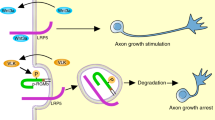
Abstract
The establishment of a polarized morphology is an essential step in the differentiation of neurons with a single axon and multiple dendrites. In cultured rat hippocampal neurons, one of several initially indistinguishable neurites is selected to become the axon. Both phosphatidylinositol 3,4,5-trisphosphate and the evolutionarily conserved Par complex (comprising Par3, Par6 and an atypical PKC (aPKC) such as PKCλ or PKCζ) are involved in axon specification. However, the initial signals that establish cellular asymmetry and the pathways that subsequently translate it into structural changes remain to be elucidated. Here we show that localization of the GTPase Rap1B to the tip of a single neurite is a decisive step in determining which neurite becomes the axon. Using GTPase mutants and RNA interference, we found that Rap1B is necessary and sufficient to initiate the development of axons upstream of Cdc42 and the Par complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Da Silva, J.S. & Dotti, C.G. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat. Rev. Neurosci. 3, 694–704 (2002).
Horton, A.C. & Ehlers, M.D. Neuronal polarity and trafficking. Neuron 40, 277–295 (2003).
Bradke, F. & Dotti, C.G. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr. Opin. Neurobiol. 10, 574–581 (2000).
Dotti, C.G., Sullivan, C.A. & Banker, G.A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454–1468 (1988).
Bradke, F. & Dotti, C.G. The role of local actin instability in axon formation. Science 283, 1931–1934 (1999).
Macara, I.G. Parsing the polarity code. Nat. Rev. Mol. Cell Biol. 5, 220–231 (2004).
Nishimura, T. et al. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat. Cell Biol. 6, 328–334 (2004).
Shi, S.H., Jan, L.Y. & Jan, Y.N. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 112, 63–75 (2003).
Etienne-Manneville, S. & Hall, A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCζ. Cell 106, 489–498 (2001).
Lin, D. et al. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat. Cell Biol. 2, 540–547 (2000).
Yamanaka, T. et al. PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells 6, 721–731 (2001).
Sohrmann, M. & Peter, M. Polarizing without a c(l)ue. Trends Cell Biol. 13, 526–533 (2003).
Chuckowree, J.A. & Vickers, J.C. Cytoskeletal and morphological alterations underlying axonal sprouting after localized transection of cortical neuron axons in vitro. J. Neurosci. 23, 3715–3725 (2003).
Tu, S.S. et al. Antiapoptotic Cdc42 mutants are potent activators of cellular transformation. Biochemistry 41, 12350–12358 (2002).
Caviston, J.P., Tcheperegine, S.E. & Bi, E. Singularity in budding: a role for the evolutionarily conserved small GTPase Cdc42p. Proc. Natl. Acad. Sci. USA 99, 12185–12190 (2002).
Irazoqui, J.E., Gladfelter, A.S. & Lew, D.J. Scaffold-mediated symmetry breaking by Cdc42p. Nat. Cell Biol. 5, 1062–1070 (2003).
Bito, H. et al. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron 26, 431–441 (2000).
Luo, L. et al. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature 379, 837–840 (1996).
Luo, L. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1, 173–180 (2000).
Ng, J. et al. Rac GTPases control axon growth, guidance and branching. Nature 416, 442–447 (2002).
Ruchhoeft, M.L., Ohnuma, S., McNeill, L., Holt, C.E. & Harris, W.A. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J. Neurosci. 19, 8454–8463 (1999).
Threadgill, R., Bobb, K. & Ghosh, A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron 19, 625–634 (1997).
Da Silva, J.S. et al. RhoA/ROCK regulation of neuritogenesis via profilin IIa-mediated control of actin stability. J. Cell. Biol. 162, 1267–1279 (2003).
Tsygankova, O.M., Saavedra, A., Rebhun, J.F., Quilliam, L.A. & Meinkoth, J.L. Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol. Cell. Biol. 21, 1921–1929 (2001).
Weiner, O.D. et al. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 4, 509–513 (2002).
Paddison, P.J., Caudy, A.A., Bernstein, E., Hannon, G.J. & Conklin, D.S. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 16, 948–958 (2002).
Etienne-Manneville, S. Cdc42—the centre of polarity. J. Cell Sci. 117, 1291–1300 (2004).
Bos, J.L., de Rooij, J. & Reedquist, K.A. Rap1 signalling: adhering to new models. Nat. Rev. Mol. Cell Biol. 2, 369–377 (2001).
Caron, E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J. Cell Sci. 116, 435–440 (2003).
Chant, J. Cell polarity in yeast. Annu. Rev. Cell Dev. Biol. 15, 365–391 (1999).
Park, H.O., Kang, P.J. & Rachfal, A.W. Localization of the Rsr1/Bud1 GTPase involved in selection of a proper growth site in yeast. J. Biol. Chem. 277, 26721–26724 (2002).
Pruyne, D. & Bretscher, A. Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113, 365–375 (2000).
Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–35 (2002).
Rolls, M.M. & Doe, C.Q. Cell polarity: from embryo to axon. Nature 421, 905–906 (2003).
Brewer, G.J., Torricelli, J.R., Evege, E.K. & Price, P.J. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35, 567–576 (1993).
Washbourne, P. & McAllister, A.K. Techniques for gene transfer into neurons. Curr. Opin. Neurobiol. 12, 566–573 (2002).
Bradke, F. & Dotti, C.G. Differentiated neurons retain the capacity to generate axons fro m dendrites. Curr. Biol. 10, 1467–1470 (2000).
Acknowledgements
We are grateful to R. Fiore, V. Gerke, C. Klämbt, and M. Müller for comments on the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SPP1111) and Fonds der Chemischen Industrie (A.W.P.) and a fellowship from the Boehringer Ingelheim Fonds (J.C.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Distribution of Rap1B, Par3, P-Akt, and Cdc42. (PDF 832 kb)
Supplementary Fig. 2
Rap1B localization after inhibition of neurite extension. (PDF 120 kb)
Supplementary Fig. 3
Effects of different GTPases on the extension of axons and dendrites. (PDF 638 kb)
Supplementary Fig. 4
Stage-dependent effects of Rap1B. (PDF 22 kb)
Supplementary Fig. 5
Effects of mPar3 and mPar6 expression. (PDF 113 kb)
Supplementary Fig. 6
The establishment of polarity in hippocampal neurons. (PDF 757 kb)
Supplementary Table 1
Number of axons and minor neurites. (PDF 17 kb)
Supplementary Table 2
Length of minor neurites. (PDF 18 kb)
Rights and permissions
About this article
Cite this article
Schwamborn, J., Püschel, A. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci 7, 923–929 (2004). https://doi.org/10.1038/nn1295
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1295
This article is cited by
-
The role of TSC1 and TSC2 proteins in neuronal axons
Molecular Psychiatry (2024)
-
Evaluation of BDE-47-induced neurodevelopmental toxicity in zebrafish embryos
Environmental Science and Pollution Research (2023)
-
Cdc42 Facilitates Axonogenesis by Enhancing Microtubule Stabilization in Primary Hippocampal Neurons
Cellular and Molecular Neurobiology (2021)
-
Rap1b but not Rap1a in the forebrain is required for learned fear
Cell & Bioscience (2020)
-
The Sema3A receptor Plexin-A1 suppresses supernumerary axons through Rap1 GTPases
Scientific Reports (2018)