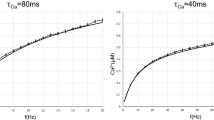
Abstract
The stability of cortical neuron activity in vivo suggests that the firing rates of both excitatory and inhibitory neurons are dynamically adjusted. Using dual recordings from excitatory pyramidal neurons and inhibitory fast-spiking neurons in neocortical slices, we report that sustained activation by trains of several hundred presynaptic spikes resulted in much stronger depression of synaptic currents at excitatory synapses than at inhibitory ones. The steady-state synaptic depression was frequency dependent and reflected presynaptic function. These results suggest that inhibitory terminals of fast-spiking cells are better equipped to support prolonged transmitter release at a high frequency compared with excitatory ones. This difference in frequency-dependent depression could produce a relative increase in the impact of inhibition during periods of high global activity and promote the stability of cortical circuits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hubel, D. H. Single unit activity in striate cortex of unrestrained cats. J. Physiol. (Lond.) 147, 226–238 (1959).
Mountcastle, V. B., Talbot, W. H., Sakata, H. & Hyvärinen, J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J. Neurophysiol. 32, 452–458 (1969).
Hubel, D. H. & Wiesel, T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. (Lond.) 160, 106–154 ( 1962).
Abeles, M. Corticonics. Neural Circuits of the Cerebral Cortex (Cambridge Univ. Press, Cambridge, 1991).
Azouz, R., Gray, C. M., Nowak, L. G. & McCormick, D. A. Physiological properties of inhibitory interneurons in cat striate cortex. Cereb. Cortex 7, 534–545 (1997).
White, E. L. Cortical Circuits (Birkhaüser, Boston, 1989).
Silva, L. R., Amitai, Y. & Connors, B. W. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science 251, 432–435 (1991).
Shadlen, M. N. & Newsome, W. T. Noise, neural codes and cortical organization. Curr. Opin. Neurobiol. 4, 569–579 (1994).
Buzsaki, G. & Chrobak, J. J. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr. Opin. Neurobiol. 5, 504–510 (1995).
Somers, D. C. Nelson, S. B. & Sur, M. An emergent model of orientation selectivity in cat visual cortical simple cells. J. Neurosci. 15, 5448–5465 (1995).
Douglas, R. J., Mahowald, M., Martin, K. A. & Stratford, K. J. The role of synapses in cortical computation. J. Neurocytol. 25, 893–911 (1996).
Konig, P., Engel, A. K. & Singer, W. Integrator or coincidence detector? The role of the cortical neuron revisited. Trends Neurosci. 19, 130–137 (1996).
Tsodyks, M. V. & Markram, H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc. Natl. Acad. Sci. USA 94, 719–723 (1997).
Abbott, L. F., Varela, J. A., Sen, K. & Nelson, S. B. Synaptic depression and cortical gain control. Science 275, 220–224 (1997).
Gutnick, M. J., Connors, B. W. & Prince, D. A. Mechanisms of neocortical epileptogenesis in vitro. J. Neurophysiol. 48, 1321– 1335 (1982).
Somogyi., P., Kisvárday, Z. F., Martin, K. A. & Whitteridge, D. Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of cat. Neuroscience 10, 261–294 (1983).
Kawaguchi, Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J. Neurophysiol. 69, 416–431 ( 1993).
Thomson, A. M., West, D. C., Hahn, J. & Deuchars, J. Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J. Physiol. (Lond.) 496, 81–102 (1996).
Tamás, G., Buhl, E. H. & Somogyi, P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J. Physiol. (Lond.) 500, 715–738 (1997).
Thomson, A. M., Deuchars, J. & West, D. C. Single axon excitatory postsynaptic potentials in neocortical interneurons exhibit pronounced paired pulse facilitation. Neuroscience 54, 347–360 (1993).
Thomson, A. M. Activity-dependent properties of synaptic transmission at two classes of connections made by rat neocortical pyramidal axons in vitro. J. Physiol. (Lond.) 502, 131–147 ( 1997).
Reyes, A. D. et al. Target-cell-specific facilitation and depression in neocortical circuits. Nature Neurosci. 1, 279– 285 (1998).
Markram, H., Tsodyks, M. & Wang, Y. Differential signaling via the same axon of neocortical pyramidal neurons. Proc. Natl. Acad. Sci. USA 95, 5323–5328 (1998).
Porter, J. T. et al. Properties of bipolar VIPergic interneurones and their excitation by pyramidal neurons in the rat neocortex. Eur. J. Neurosci. (in press).
Tarczy-Hornoch, K., Martin, K. A., Jack, J. J. & Stratford, K. J. Synaptic interactions between smooth and spiny neurones in layer 4 of cat visual cortex in vitro. J. Physiol. (Lond.) 508, 351–363 (1998).
Zhang, S. & Trussell, L. O. Voltage clamp analysis of excitatory synaptic transmission in the avian nucleus magnocellularis. J. Physiol. (Lond.) 480, 123–136 (1994).
Takahashi, M., Kovalchuk, Y. & Attwell, D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. J. Neurosci. 15, 5693– 5702 (1995).
Larkman, A. U., Jack, J. J. & Stratford, K. J. Quantal analysis of excitatory synapses in rat hippocampal CA1 in vitro during low-frequency depression. J. Physiol. (Lond.) 505, 457–471 ( 1997).
Partin, K. M., Patneau, D. K., Winters, C. A., Mayer, M. L. & Buonanno, A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron 11, 1069–1082 ( 1993).
Pouzat, C. & Hestrin, S. Developmental regulation of basket/stellate cell→Purkinje cell synapses in the cerebellum. J. Neurosci. 17, 9104–9112 ( 1997).
Katz, B. Nerve, Muscle, and Synapse (McGraw Hill, New York, 1966).
Dobrunz, L. E. & Stevens, C. F. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18, 995–1008 ( 1997).
Liu, G. & Tsien, R. W. Properties of synaptic transmission at single hippocampal synaptic boutons. Nature 375, 404–408 (1995).
Ryan, T. A. & Smith, S. J. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron 14, 983–989 (1995).
Stevens, C. F. & Tsujimoto, T. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc. Natl. Acad. Sci. USA 92, 846–849 (1995).
Shupliakov, O. et al. Synaptic vesicle endocytosis impaired by disruption of dynamin-H3 domain interactions. Science 276, 259–263 (1997).
von Gersdorff, H. & Matthews, G. Depletion and replenishment of vesicle pools at a ribbon-type synaptic terminal. J. Neurosci. 17, 1919–1927 (1997).
Neher, E. Vesicle pools and Ca2+ microdomains: New tools for understanding their roles in neurotransmitter release. Neuron 20, 389–399 (1998).
Wu, L. G. & Betz, W. J. Kinetics of synaptic depression and vesicle recycling after tetanic stimulation of frog motor nerve terminals. Biophys. J. 74, 3003–3009 (1998).
Klingauf, J, Kavalali, E. T. & Tsien, R. W. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature 394, 581– 585 (1998).
Silver, R. A., Momiyama, A. & Cull-Candy, S. G. Locus of frequency-dependent depression identified with multiple-probability fluctuation analysis at rat climbing fibre–Purkinje cell synapses. J. Physiol. (Lond.) 510, 881–902 (1998).
Wang, C. & Zucker, R. S. Regulation of synaptic vesicle recycling by calcium and serotonin. Neuron 21, 155–167 (1998).
Zucker, R. S. Short-term synaptic plasticity. Annu. Rev. Neurosci. 12, 13–31 (1989).
Forsythe, I. D., Tsujimoto, T., Barnes-Davies, M., Cuttle, M. F. & Takahashi, T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron 20, 797–807 ( 1998).
Chance, F. S., Nelson, S. B. & Abbott, L. F. Synaptic depression and the temporal response characteristics of V1 cells. J. Neurosci. 18, 4785– 4799 (1998).
Ohzawa, I., Sclar, G. & Freeman, R. D. Contrast gain control in the cat's visual system. J. Neurophysiol. 54, 651– 667 (1985).
Moshon, J. A., Thompson, I. D. & Tolhurst, D. J. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J. Physiol. (Lond.) 283, 101–120 ( 1978).
Hawken, M. J., Shapley, R. M. & Grosof, D. H. Temporal-frequency selectivity in monkey visual cortex. Vis. Neurosci. 13, 477– 492 (1996).
Carandini, M. & Ferster, D. A tonic hyperpolarization underlying contrast adaptation in cat visual cortex. Science 276 , 949–952 (1997).
Markram, H., Lübke, J., Frotscher, M., Roth, A. & Sakmann, B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J. Physiol. (Lond.) 500, 409– 440 (1997).
Acknowledgements
We thank Jeffry Isaacson and Pankaj Sah for their comments on the manuscript. This work was supported by NIH grant (S.H.) and a grant from the Spanish Ministry of Education and Science (M.G.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galarreta, M., Hestrin, S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat Neurosci 1, 587–594 (1998). https://doi.org/10.1038/2822
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/2822
This article is cited by
-
Electrophysiology of ionotropic GABA receptors
Cellular and Molecular Life Sciences (2021)
-
Short-term dynamics of input and output of CA1 network greatly differ between the dorsal and ventral rat hippocampus
BMC Neuroscience (2019)
-
Synaptotagmin 7 confers frequency invariance onto specialized depressing synapses
Nature (2017)
-
Neural field model of seizure-like activity in isolated cortex
Journal of Computational Neuroscience (2017)
-
A neural mass model of phase–amplitude coupling
Biological Cybernetics (2016)