Abstract
Morphine is a powerful analgesic for severe pain, and this effect is thought to be mediated by inhibitory G-protein coupled receptors (GPCRs). Here we show that morphine activates TrkB in a NT-4–dependent manner and provide evidence from transgenic mice that such activation partially mediates morphine-induced analgesia. These findings show that the anti-nociceptive effect of morphine is partially mediated by NT-4–induced TrkB receptor activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Law, P.Y., Wong, Y.H. & Loh, H.H. Annu. Rev. Pharmacol. Toxicol. 40, 389–430 (2000).
Luttrell, L.M., Daaka, Y. & Lefkowitz, R.J. Curr. Opin. Cell Biol. 11, 177–183 (1999).
Lee, F.S. & Chao, M.V. Proc. Natl. Acad. Sci. USA 98, 3555–3560 (2001).
McLaughlin, J.P. & Chavkin, C. Mol. Pharmacol. 59, 1360–1368 (2001).
Appleyard, S.M., McLaughlin, J.P. & Chavkin, C. J. Biol. Chem. 275, 38281–38285 (2000).
Yan, Q. et al. J. Comp. Neurol. 378, 135–157 (1997).
Ding, Y.Q., Kaneko, T., Nomura, S. & Mizuno, N. J. Comp. Neurol. 367, 375–402 (1996).
Middlemas, D.S., Meisenhelder, J. & Hunter, T. J. Biol. Chem. 269, 5458–5466 (1994).
Guiton, M., Gunn-Moore, F.J. & Tavare, J.M. Biochem. Soc. Trans. 23, 176S (1995).
Segal, R.A. et al. J. Biol. Chem. 271, 20175–20181 (1996).
Patapoutian, A. & Reichardt, L.F. Curr. Opin. Neurobiol. 11, 272–280 (2001).
Saarelainen, T. et al. Mol. Cell. Neurosci. 16, 87–96 (2000).
Minichiello, L. et al. Neuron 21, 335–345 (1998).
Siuciak, J.A., Altar, C.A., Wiegand, S.J. & Lindsay, R.M. Brain Res. 633, 326–330 (1994).
Acknowledgements
We thank D. Kaplan for TrkB antibody and M. Malcangio for advice on NT-4 release assay. Supported by Biotechnology Program of the EU QLG3-1999-00602, Swedish Medical Research Council, Swedish Cancer Society and Göran Gustafssons Foundation (P.E.); fellowship from David and Astrid Hageléns Foundation (G.L.); Academy of Finland, TEKES and Sigrid Juselius Foundation (E.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1.
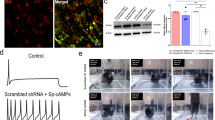
Morphine induced phosphoTrk immunoreactivity in brainstem nuclei. Confocal images of the trigeminal spinal nucleus and locus coeruleus (insets) neurons stained with anti-Trk autophosphorylation antibody 30 min after (a) morphine (10 mg/kg), (b) morphine (10 mg/kg) followed by naloxone (1 mg/kg), and (c) naloxone alone. Note increased immunoreactivity in morphine but not morphine plus naloxone or naloxone treated mice. Arrows indicate phospho-Trk positive cells in the spinal trigeminal nucleus. Scale bar, 200 μm for the trigeminal spinal nucleus and 75 μm for the locus coeruleus. Methods: C57/BL6 mice were treated with morphine (10 mg/kg, s.c.; n = 3), or with morphine (10 mg/kg, s.c.) followed by naloxone (1 mg/kg, i.p. n = 2) 10 min later, or only naloxone (1 mg/kg, i.p. n = 1). Thirty minutes after morphine treatment or 10 min after naloxone administration, mice were anesthetized by isoflurane (5% [v/v%] in 70% N2O and 30% O2) and transcardially perfused with ice-cold physiological saline (15 ml) that was followed by 80 ml of fixative composed of 4% paraformaldehyde, 0.1% glutaraldehyde and 1mM sodium orthovanadate in sodium phosphate buffer (PB, 0.1M, pH 7.4) at a flow rate of 4 - 4.5 ml/min. Whole brains were removed from the skulls and post-fixed in 4% paraformaldehyde and 1mM sodium orthovanadate in PB overnight. Brains were then cryoprotected in 30% sucrose in physiological saline for 24 hrs, cryostat sectioned at a 40-μm thickness, and collected in 0.1M PB with 0.1% Na-azide as preservative. Series of free-floating sections of all animals were processed simultaneously in a uniform manner. After extensive rinsing in PB, sections were pre-incubated in a mixture of 10% normal donkey serum (NDS, Jackson ImmunoResearch, USA), 5% normal bovine serum (BSA) and 0.1% triton X-100 in PB for 60 min. Subsequently, sections were exposed to the primary antibody rabbit anti-Tyr674,Tyr675-phosphorylated tyrosine kinase (1:25; Calbiochem, USA) in PB containing 1% NDS, 0.1% BSA and 0.1% triton X-100 for 48 hrs at 4 °C under continuous agitation of the incubation medium. Thereafter, sections were washed in repeated changes of PB and incubated with carbocyanine 3-conjugated donkey anti-rabbit secondary antibody (1:200; affinity-pure for multiple labeling without cross-reactivity with mouse serum proteins, Jackson) in PB containing 2% BSA for 2 hrs at room temperature. Next, sections were rinsed in PB, dipped in distilled water and mounted on fluorescence-free glasses. Sections were coverslipped using Entellan (in toluene). Sections were analyzed using confocal laser-scanning microscopy with appropriate excitation and emission filters (543 nm and 560-610 nm, respectively; Zeiss 510, Germany). Selected images were captured using identical pinhole, detector gain, detector offset, amplification gain and scanning control settings, where specimen of morphine-treated animals were used for calibration of scanning conditions. Subsequently, multi-panel plates of unmodified images were created employing Paint Shop Pro (v. 7.01, Jasc Inc., USA) and Adobe Photoshop (v. 6.0, Adobe Inc., USA). Scanning data: pinhole: 60 μm, (less than 1.9 micrometer optical slice), adjusted for even light distribution. Detector gain: 960; offset: -0.3; amplificator gain: 1; 100% laser excitation on 543 nm channel; speed: 7, lines: 4; zoom: (spinal trigeminal nucleus images): 1, Locus coeruleus images: 1.5. (JPG 48 kb)
Supplementary Fig. 2.
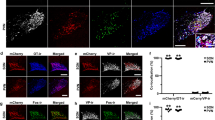
Measurements of morphine and morphine metabolites in the plasma of NT-4-/- and NT-4+/+ mice. Mice were decapitated 30 minutes after morphine administration (10 mg/kg, s.c.) and blood samples (300 μl) collected in ice-chilled tubes containing heparin (10 IU/ml) were centrifuged for separation of the plasma. Morphine (a), Morphine-6-glucuronide (b), and Morphine-3 glucuronide (c) were measured by a reverse phase HPLC column (C18, Spherisorb S3 ODS2 100 x 4 mm I.D.; 3 μm particles) and no difference was observed between wild-type (n = 5) and NT-4-/- (n = 6) mice (Student t-test). This shows that the difference in morphine response between mutated mice and wild-type mice is not caused by a difference in metabolisation of morphine. (JPG 34 kb)
Rights and permissions
About this article
Cite this article
Lucas, G., Hendolin, P., Harkany, T. et al. Neurotrophin-4 mediated TrkB activation reinforces morphine-induced analgesia. Nat Neurosci 6, 221–222 (2003). https://doi.org/10.1038/nn1021
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1021
This article is cited by
-
Pharmacologically Diverse Antidepressants Rapidly Activate Brain-Derived Neurotrophic Factor Receptor TrkB and Induce Phospholipase-Cγ Signaling Pathways in Mouse Brain
Neuropsychopharmacology (2007)
-
Pain Facilitation and Activity-Dependent Plasticity in Pain Modulatory Circuitry: Role of BDNF-TrkB Signaling and NMDA Receptors
Molecular Neurobiology (2007)
-
Distinctive Profiles of Gene Expression in the Human Nucleus Accumbens Associated with Cocaine and Heroin Abuse
Neuropsychopharmacology (2006)