Abstract
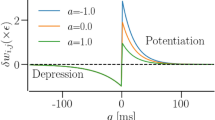
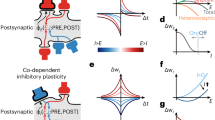
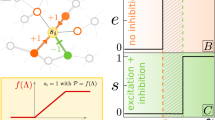
The faithful production of rhythms by many neural circuits depends critically on the strengths of inhibitory synaptic connections. We propose a model in which the strengths of inhibitory synapses in a central pattern-generating circuit are subject to activity-dependent plasticity. The strength of each synapse is modified as a function of the global activity of the postsynaptic neuron and by correlated activity of the pre- and postsynaptic neurons. This allows the self-assembly, from random initial synaptic strengths, of two cells into reciprocal oscillation and three cells into a rhythmic triphasic motor pattern. This self-assembly illustrates that complex oscillatory circuits that depend on multiple inhibitory synaptic connections can be tuned via simple activity-dependent rules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Marder, E. & Calabrese, R. L. Principles of rhythmic motor pattern generation. Physiol. Rev. 76, 687–717 (1996).
Brown, T. G. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J. Physiol. (Lond.) 48, 18–46 (1914).
Grillner, S., Parker, D. & el Manira, A. Vertebrate locomotion—a lamprey perspective. Ann. NY Acad. Sci. 860, 1–18 (1998).
Sillar, K. T., Reith, C. A. & McDearmid, J. R. in Neuronal Mechanisms for Generating Locomotor Activity Vol. 860 (eds. Kiehn, O., Harris-Warrick, R. M., Jordan, L. M., Hultborn, H. & Kudo, N.) 318–332 (New York Academy of Sciences, New York, 1998).
Arbas, E. A. & Calabrese, R. L. Slow oscillations of membrane potential in interneurons that control heartbeat in the medicinal leech. J. Neurosci. 7, 3953–3960 (1987).
Angstadt, J. D. & Calabrese, R. L. Calcium currents and graded synaptic transmission between heart interneurons of the leech. J. Neurosci. 11, 746–759 (1991).
Nadim, F., Olsen, Ø. H., De Schutter, E. & Calabrese, R. L. Modeling the leech heartbeat elemental oscillator. I. Interactions of intrinsic and synaptic currents. J. Comput. Neurosci. 2, 215–235 (1995).
Olsen, Ø. H., Nadim, F. & Calabrese, R. L. Modeling the leech heartbeat elemental oscillator. II. Exploring the parameter space. J. Comput. Neurosci. 2, 237–257 (1995).
Olsen, Ø. H. & Calabrese, R. L. Activation of intrinsic and synaptic currents in leech heart interneurons by realistic waveforms. J. Neurosci. 16, 4958–4970 (1996).
Satterlie, R. A. Reciprocal inhibition and postinhibitory rebound produce reverberation in a locomotor pattern generator. Science 229, 402–404 (1985).
Friesen, W. O. Reciprocal inhibition: a mechanism underlying oscillatory animal movements. Neurosci. Biobehav. 18, 547–553 (1994).
Hartline, D. K. & Gassie, D. V. Jr. Pattern generation in the lobster (Panulirus) stomatogastric ganglion. I. Pyloric neuron kinetics and synaptic interactions. Biol. Cybern. 33, 209–222 (1979).
Harris-Warrick, R. M., Coniglio, L. M., Barazangi, N., Guckenheimer, J. & Gueron, S. Dopamine modulation of transient potassium current evokes phase shifts in a central pattern generator network. J. Neurosci. 15, 342–358 (1995).
Harris-Warrick, R. M., Coniglio, L. M., Levini, R. M., Gueron, S. & Guckenheimer, J. Dopamine modulation of two subthreshold currents produces phase shifts in activity of an identified motoneuron. J. Neurophysiol. 74, 1404–1420 (1995).
Miller, J. P. & Selverston, A. I. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. IV. Network properties of pyloric system. J. Neurophysiol. 48, 1416–1432 (1982).
Eisen, J. S. & Marder, E. A mechanism for production of phase shifts in a pattern generator. J. Neurophysiol. 51, 1375–1393 (1984).
Marty, A. & Llano, I. Modulation of inhibitory synapses in the mammalian brain. Curr. Opin. Neurobiol. 5, 335–341 (1995).
Korn, H., Oda, Y. & Faber, D. S. Long-term potentiation of inhibitory circuits and synapses in the central nervous system. Proc. Natl. Acad. Sci. USA 89, 440–443 (1992).
Oda, Y., Charpier, S., Murayama, Y., Suma, C. & Korn, H. Long-term potentiation of glycinergic inhibitory synaptic transmission. J. Neurophysiol. 74, 1056–1074 (1995).
Oda, Y., Kawasaki, K., Morita, M., Korn, H. & Matsui, H. Inhibitory long-term potentiation underlies auditory conditioning of goldfish escape behaviour. Nature 394, 182–185 (1998).
Komatsu, Y. & Iwakiri, M. Long-term modification of inhibitory synaptic transmission in developing visual cortex. Neuroreport 4, 907–910 (1993).
Komatsu, Y. GABAB receptors, monoamine receptors, and postsynaptic inositol trisphosphate-induced Ca2+ release are involved in the induction of long-term potentiation at visual cortical inhibitory synapses. J. Neurosci. 16, 6342–6352 (1996).
Aizenman, C. D., Manis, P. B. & Linden, D. J. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron 21, 827–835 (1998).
Harris-Warrick, R. M., Marder, E., Selverston, A. I. & Moulins, M. (eds.) Dynamic Biological Networks: The Stomatogastric Nervous System (MIT Press, Cambridge, Massachusetts, 1992).
Graubard, K., Raper, J. A. & Hartline, D. K. Graded synaptic transmission between spiking neurons. Proc. Natl. Acad. Sci. USA 77, 3733–3735 (1980).
Graubard, K., Raper, J. A. & Hartline, D. K. Graded synaptic transmission between identified spiking neurons. J. Neurophysiol. 50, 508–521 (1983).
Morris, C. & Lecar, H. Voltage oscillations in the barnacle giant muscle fiber. Biophys. J. 35, 193–213 (1981).
Skinner, F. K., Kopell, N. & Marder, E. Mechanisms for oscillation and frequency control in reciprocal inhibitory model neural networks. J. Comput. Neurosci. 1, 69–87 (1994).
Wang, X.-J. & Rinzel, J. Alternating and synchronous rhythms in reciprocally inhibitory model neurons. Neural Comp. 4, 84–97 (1992).
LeMasson, G., Marder, E. & Abbott, L. F. Activity-dependent regulation of conductances in model neurons. Science 259, 1915–1917 (1993).
Liu, Z., Golowasch, J., Marder, E. & Abbott, L. F. A model neuron with activity-dependent conductances regulated by multiple calcium sensors. J. Neurosci. 18, 2309–2320 (1998).
Siegel, M., Marder, E. & Abbott, L. F. Activity-dependent current distributions in model neurons. Proc. Natl. Acad. Sci. USA 91, 11308–11312 (1994).
Hartline, D. K., Russell, D. F., Raper, J. A. & Graubard, K. Special cellular and synaptic mechanisms in motor pattern generation. Comp. Biochem. Physiol. 91C, 115–131 (1988).
Tierney, A. J. & Harris-Warrick, R. M. Physiological role of the transient potassium current in the pyloric circuit of the lobster stomatogastric ganglion. J. Neurophysiol. 67, 599–609 (1992).
Richards, K. S., Miller, W. L. & Marder, E. Maturation of the rhythmic activity produced by the stomatogastric ganglion of the lobster, Homarus americanus. J. Neurophysiol. 82, 2006–2009 (1999).
Casasnovas, B. & Meyrand, P. Functional differentiation of adult neural circuits from a single embryonic network. J. Neurosci. 15, 5703–5718 (1995).
O'Donovan, M. J. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr. Opin. Neurobiol. 9, 94–104 (1999).
Golowasch, J., Casey, M., Abbott, L. F. & Marder, E. Network stability from activity-dependent regulation of neuronal conductances. Neural Comp. 11, 1079–1096 (1999).
Acknowledgements
This research was supported by MH 46742, NS 07292 and the Sloan and Keck Foundations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soto-Treviño, C., Thoroughman, K., Marder, E. et al. Activity-dependent modification of inhibitory synapses in models of rhythmic neural networks. Nat Neurosci 4, 297–303 (2001). https://doi.org/10.1038/85147
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/85147
This article is cited by
-
Categorizing Phenotypic Plasticity: An Analysis of Its Role in Human Cognitive Evolution
Journal for General Philosophy of Science (2022)
-
Burst synchronization in a scale-free neuronal network with inhibitory spike-timing-dependent plasticity
Cognitive Neurodynamics (2019)
-
Pharmacological modulation of Kv3.1 mitigates auditory midbrain temporal processing deficits following auditory nerve damage
Scientific Reports (2017)
-
Synaptic convergence regulates synchronization-dependent spike transfer in feedforward neural networks
Journal of Computational Neuroscience (2017)
-
Homeostasis of Brain Dynamics in Epilepsy: A Feedback Control Systems Perspective of Seizures
Annals of Biomedical Engineering (2009)