Abstract
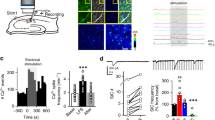
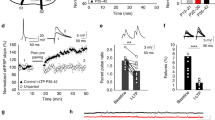
We demonstrate a form of long-term depression (LTD) in the perirhinal cortex that relies on interaction between different glutamate receptors. Group II metabotropic glutamate (mGlu) receptors facilitated group I mGlu receptor-mediated increases in intracellular calcium. This facilitation plus NMDA receptor activation may be necessary for induction of LTD at resting membrane potentials. However, depolarization enhanced NMDA receptor function and removed the requirement of synergy between group I and group II mGlu receptors: under these conditions, activation of only NMDA and group I mGlu receptors was required for LTD. Such glutamate receptor interactions potentially provide new rules for synaptic plasticity. These forms of LTD occur in the perirhinal cortex, where long-term decreases in neuronal responsiveness may mediate recognition memory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Brown, M. W. & Xiang, J.-Z. Recognition memory: neuronal substrates of the judgement of prior occurrence. Prog. Neurobiol. 55, 149–189 (1998).
Meunier, M., Bachevalier, J., Mishkin, M. & Murray, B. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys J. Neurosci. 13, 5418–5432 (1993).
Sakai, K. & Miyashita, Y. Neural organization for the long-term memory of paired associates. Nature 354, 152–155 (1999).
Corodimas, K. P. & Ledoux, J.E. Disruptive effects of posttraining perirhinal cortex lesions on conditioned fear: contributions of contextual cues Behav. Neurosci. 109, 613–619 (1995).
Zhu, X. O. & Brown, M.W. Changes in neuronal activity related to the repetition and relative familiarity of visual stimuli in rhinal and adjacent cortex of the anesthetized rat. Brain Res. 689, 101–110 (1995).
Zhu, X. O., McCabe, B. J., Aggleton, J. P. & Brown, M.W. Mapping visual recognition memory through expression of the immediate early gene c-fos. Neuroreport 7, 1871– 1875 (1996).
Bear, M. F. & Malenka, R.C. Synaptic plasticity: LTP and LTD . Curr. Opin. Neurobiol. 4, 389– 399 (1994).
Kerr, D. S. & Abraham, W.C. LTD: Many means to how many ends? Hippocampus 6, 30–34 (1996).
Bear, M. F. & Abraham, W.C. Long-term depression in hippocampus . Annu. Rev. Neurosci. 19, 437– 462 (1996).
Fujii, S., Saito, K., Miyakawa, H., Ito, K. & Kato, H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 555, 112–122 (1991).
Mulkey, R. M. & Malenka, R.C. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron 9, 967–975 ( 1992).
Dudek, S. M. & Bear, M.F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc. Natl. Acad. Sci. USA 89, 4363–4367 (1992).
Bashir, Z. I. & Collingridge, G.L. An investigation of depotentiation of long-term potentiation in the CA1 region of the hippocampus. Exp. Brain Res. 100, 437–443 (1994).
Bolshakov, V. Y. & Siegelbaum, S.A. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science 264, 1148–1152 ( 1994).
Oliet, S. H. R., Malenka, R. C. & Nicoll, R.A. Two distinct form of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron 18, 969–982 (1997).
Otani, S. & Connor, J.A. Requirement of rapid Ca2+ entry and synaptic activation of metabotropic glutamate receptors for the induction of long-term depression in adult rat hippocampus. J. Physiol. (Lond.) 511, 761–770 (1998).
Fitzjohn, S. M. et al. The potent mGlu receptor antagonist LY341495 identifies roles for both cloned and novel mGlu receptors in hippocampal synaptic plasticity . Neuropharmacology 37, 1445– 1458 (1998).
Mulkey, R. M., Herron, C. E. & Malenka, R.C. An essential role for protein phosphatases in hippocampal long-term depression. Science 261, 1051– 1055 (1993).
Pin, J.-P. & Duvoisin, R. The metabotropic glutamate receptors: Structure and functions. Neuropharmacology 34, 1–26 (1995).
Conn, P. J. & Pin, J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 37, 205–237 (1997).
Anwyl, R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res. Rev. 29, 83– 120 (1999).
Reyes, M. & Stanton, P.K. Induction of hippocampal long-term depression requires release of Ca2+ from separate presynaptic and postsynaptic intracellular stores. J. Neurosci. 16, 5951–5960 (1996).
Yokoi, M. et al. Impairment of hippocampal mossy fibre LTD in mice lacking mGluR2 . Science 273, 645–647 (1996).
Domenici, M. R., Berretta, N. & Cherubini, E. Two distinct forms of long-term depression coexist at the mossy fiber-CA3 synapse in the hippocampus during development. Proc. Natl. Acad. Sci. USA 95, 8310– 8315 (1998).
Tzounopoulos, T., Janz, R., Südhof, T. C., Nicoll, R. A. & Malenka, R.C. A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron 21, 837–845 (1998).
Huang, L. Q., Rowan, M. J. & Anwyl, R. mGluR II agonist inhibition of LTP induction, and mGluR II antagonist inhibition of LTD induction, in the dentate gyrus in vitro . Neuroreport 8, 687– 693 (1997).
Manahan-Vaughan, D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J. Neurosci. 17, 3303– 3311 (1997).
Manahan-Vaughan, D. Priming of group 2 metabotropic glutamate receptors facilitates induction of long-term depression in the dentate gyrus of freely moving rats. Neuropharmacology 37, 1459–1464 (1998).
Bilkey, D.K. Long-term potentiation in the in vitro perirhinal cortex displays associative properties. Brain Res. 733, 297– 300 (1996).
Ziakopoulos, Z., Tillet, C. W., Brown, M. W. & Bashir, Z.I. Input- and layer-dependent synaptic plasticity in the rat perirhinal cortex in vitro. Neuroscience 92, 459– 472 (1999).
Eaton, S. A. et al. Competitive antagonism at metabotropic glutamate receptors by (S)-4-carboxyphenylglycine and (R,S)-α-methyl-4-carboxyphenylglycine . Eur. J. Pharmacol. 244, 195– 197 (1993).
Bashir, Z. I. et al. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature 363, 347–350 (1993).
Pellicciari, R. et al. 1-aminoindan-1,5-dicarboxylic acid: a novel antagonist at phosholipase C-linked metabotropic glutamate receptors. J. Med. Chem. 38, 3717–3719 ( 1995).
Moroni, F. et al. Pharmacological characterization of 1-aminoindan-1,5-dicarboxylic acid, a potent mGluR1 antagonist. J. Pharmacol. Exp. Ther. 281, 721–729 (1997).
Salt, T. E. & Eaton, S.A. Distinct presynaptic metabotropic receptors for l-AP4 and CCG1 on GABAergic terminals: pharmacological evidence using novel α-methyl derivative mGluR antagonists, MAP4 and MCCG, in the rat thalamus in vivo. Neuroscience 65, 5–13 (1995).
McCaffery, B. et al. Synaptic depression induced by pharmacological activation of metabotropic glutamate receptors in the perirhinal cortex in vitro. Neuroscience 93, 977–984 (1999).
Ito, I. et al. 3,5-Dihydroxyphenylglycine: a potent agonist of metabotropic glutamate receptors. Neuroreport 3, 1013– 1016 (1992).
Wilsch, V. W., Pidoplichko, V. I., Opitz, T., Shinozaki, H. & Reymann, K.G. Metabotropic glutamate receptor agonist DCG-IV as NMDA receptor agonist in immature rat hippocampal neurones . Eur. J. Pharmacol. 262, 287– 291 (1994).
Scanziani, M., Salin, P. A., Vogt, K. E., Malenka, R. C. & Nicoll, R.A. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature 385, 630–634 ( 1997).
Schoepp, D. D. et al. The novel metabotropic glutamate receptor agonist 2R,4 R- APDC potentiates stimulation of phosphoinositide hydrolysis in the rat hippocampus by 3,5–dihydroxyphenylglycine: Evidence for a synergistic interaction between group 1 and group 2 receptors. Neuropharmacology 35, 1661–1672 ( 1996).
Mistry, R., Golding, N. & Challiss, R.A.J. Regulation of phosphoinositide turnover in neonatal rat cerebral cortex by group I- and II-selective metabotropic glutamate receptor agonists. Br. J. Pharmacol. 123, 581– 589 (1998).
Noel, J. et al. Surface expression of AMPA receptors in hippocampal neurones is regulated by an NSF-dependent mechanisms. Neuron 23, 365–376 (1999).
Doherty, A. J., Collingridge, G. L. & Jane, D.E. Antagonist activity of α-substituted 4-carboxyphenylglycine analogues at group I metabotropic glutamate receptors expressed in CHO cells . Br. J. Pharmacol. 126, 205– 210 (1999).
Burwell, R. D., Witter, M. P. & Amaral, D.G. Perirhinal and postrhinal cortices of the rat: A review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus 5, 390– 408 (1995).
Acknowledgements
We thank A. Doherty, L. Pickard and V. Collett for help with cell culture and calcium imaging and G.L. Collingridge for discussions. This work was supported by the BBSRC, MRC and Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, K., Kemp, N., Noel, J. et al. A new form of long-term depression in the perirhinal cortex. Nat Neurosci 3, 150–156 (2000). https://doi.org/10.1038/72093
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/72093
This article is cited by
-
MK-801 and cognitive functions: Investigating the behavioral effects of a non-competitive NMDA receptor antagonist
Psychopharmacology (2023)
-
Modulating role of serotonergic signaling in sleep and memory
Pharmacological Reports (2022)
-
Muscarinic Receptor-Dependent Long Term Depression in the Perirhinal Cortex and Recognition Memory are Impaired in the rTg4510 Mouse Model of Tauopathy
Neurochemical Research (2019)
-
Effects of the Positive Allosteric Modulator of Metabotropic Glutamate Receptor 5, VU-29, on Impairment of Novel Object Recognition Induced by Acute Ethanol and Ethanol Withdrawal in Rats
Neurotoxicity Research (2018)
-
Mechanisms of memory storage in a model perirhinal network
Brain Structure and Function (2017)