Abstract
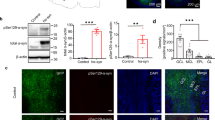
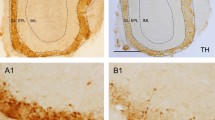
The prion protein PrPC is infamous for its role in disease, but its normal physiological function remains unknown. Here we found a previously unknown behavioral phenotype of Prnp−/− mice in an odor-guided task. This phenotype was manifest in three Prnp knockout lines on different genetic backgrounds, which provides strong evidence that the phenotype is caused by a lack of PrPC rather than by other genetic factors. Prnp−/− mice also showed altered behavior in a second olfactory task, suggesting that the phenotype is olfactory specific. Furthermore, PrPC deficiency affected oscillatory activity in the deep layers of the main olfactory bulb, as well as dendrodendritic synaptic transmission between olfactory bulb granule and mitral cells. Notably, both the behavioral and electrophysiological alterations found in Prnp−/− mice were rescued by transgenic neuronal-specific expression of PrPC. These data suggest that PrPC is important in the normal processing of sensory information by the olfactory system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bueler, H. et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356, 577–582 (1992).
Bueler, H. et al. Mice devoid of PrP are resistant to scrapie. Cell 73, 1339–1347 (1993).
Steele, A.D., Lindquist, S. & Aguzzi, A. The prion protein knockout mouse, a phenotype under challenge. Prion 1, 83–93 (2007).
Tobler, I. et al. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature 380, 639–642 (1996).
Tobler, I., Deboer, T. & Fischer, M. Sleep and sleep regulation in normal and prion protein–deficient mice. J. Neurosci. 17, 1869–1879 (1997).
Criado, J.R. et al. Mice devoid of prion protein have cognitive deficits that are rescued by reconstitution of PrP in neurons. Neurobiol. Dis. 19, 255–265 (2005).
Rangel, A. et al. Enhanced susceptibility of Prnp-deficient mice to kainate-induced seizures, neuronal apoptosis and death: role of AMPA/kainate receptors. J. Neurosci. Res. 85, 2741–2755 (2007).
Walz, R. et al. Increased sensitivity to seizures in mice lacking cellular prion protein. Epilepsia 40, 1679–1682 (1999).
Ford, M.J. et al. A marked disparity between the expression of prion protein and its message by neurones of the CNS. Neuroscience 111, 533–551 (2002).
Le Pichon, C.E. & Firestein, S. Expression and localization of the prion protein PrPC in the olfactory system of the mouse. J. Comp. Neurol. 508, 487–499 (2008).
Kay, L.M. & Stopfer, M. Information processing in the olfactory systems of insects and vertebrates. Semin. Cell Dev. Biol. 17, 433–442 (2006).
Schoppa, N.E. & Urban, N.N. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 26, 501–506 (2003).
Walz, A., Mombaerts, P., Greer, C.A. & Treloar, H.B. Disrupted compartmental organization of axons and dendrites within olfactory glomeruli of mice deficient in the olfactory cell adhesion molecule, OCAM. Mol. Cell. Neurosci. 32, 1–14 (2006).
Wong, S.T. et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27, 487–497 (2000).
Trinh, K. & Storm, D.R. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat. Neurosci. 6, 519–525 (2003).
Moore, R.C. et al. Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J. Mol. Biol. 292, 797–817 (1999).
Omri, B. et al. The Lck tyrosine kinase is expressed in brain neurons. J. Neurochem. 67, 1360–1364 (1996).
Schellinck, H.M., Price, S.R. & Wong, M.J. Using ethologically relevant tasks to study olfactory discrimination in rodents. in Chemical Signals in Vertebrates II (eds. Hurst, J.L., Beynon, R.J., Roberts, S.C. & Wyatt, T.D.) (Springer, New York, 2008).
Lagier, S. et al. GABAergic inhibition at dendrodendritic synapses tunes gamma oscillations in the olfactory bulb. Proc. Natl. Acad. Sci. USA 104, 7259–7264 (2007).
Schoppa, N.E. Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron 49, 271–283 (2006).
Mori, K. & Takagi, S.F. An intracellular study of dendrodendritic inhibitory synapses on mitral cells in the rabbit olfactory bulb. J. Physiol. (Lond.) 279, 569–588 (1978).
Nicoll, R.A. Inhibitory mechanisms in the rabbit olfactory bulb: dendrodendritic mechanisms. Brain Res. 14, 157–172 (1969).
Lledo, P.M. & Lagier, S. Adjusting neurophysiological computations in the adult olfactory bulb. Semin. Cell Dev. Biol. 17, 443–453 (2006).
Stopfer, M. Olfactory processing: massive convergence onto sparse codes. Curr. Biol. 17, R363–R364 (2007).
Kashiwadani, H., Sasaki, Y.F., Uchida, N. & Mori, K. Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. J. Neurophysiol. 82, 1786–1792 (1999).
Beshel, J., Kopell, N. & Kay, L.M. Olfactory bulb gamma oscillations are enhanced with task demands. J. Neurosci. 27, 8358–8365 (2007).
Brown, S.L., Joseph, J. & Stopfer, M. Encoding a temporally structured stimulus with a temporally structured neural representation. Nat. Neurosci. 8, 1568–1576 (2005).
Nusser, Z., Kay, L.M., Laurent, G., Homanics, G.E. & Mody, I. Disruption of GABA(A) receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. J. Neurophysiol. 86, 2823–2833 (2001).
Urban, N.N. Lateral inhibition in the olfactory bulb and in olfaction. Physiol. Behav. 77, 607–612 (2002).
Yokoi, M., Mori, K. & Nakanishi, S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc. Natl. Acad. Sci. USA 92, 3371–3375 (1995).
Shepherd, G.M. The Synpatic Organization of the Brain (Oxford University Press, New York, 2003).
Collinge, J. et al. Prion protein is necessary for normal synaptic function. Nature 370, 295–297 (1994).
Lledo, P.M., Tremblay, P., DeArmond, S.J., Prusiner, S.B. & Nicoll, R.A. Mice deficient for prion protein exhibit normal neuronal excitability and synaptic transmission in the hippocampus. Proc. Natl. Acad. Sci. USA 93, 2403–2407 (1996).
Curtis, J., Errington, M., Bliss, T., Voss, K. & MacLeod, N. Age-dependent loss of PTP and LTP in the hippocampus of PrP-null mice. Neurobiol. Dis. 13, 55–62 (2003).
Fournier, J.G., Escaig-Haye, F. & Grigoriev, V. Ultrastructural localization of prion proteins: physiological and pathological implications. Microsc. Res. Tech. 50, 76–88 (2000).
Spielhaupter, C. & Schatzl, H.M. PrPC directly interacts with proteins involved in signaling pathways. J. Biol. Chem. 276, 44604–44612 (2001).
Aguzzi, A. & Polymenidou, M. Mammalian prion biology: one century of evolving concepts. Cell 116, 313–327 (2004).
Manson, J.C. et al. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol. Neurobiol. 8, 121–127 (1994).
Lorig, T.S., Elmes, D.G., Zald, D.H. & Pardo, J.V. A computer-controlled olfactometer for fMRI and electrophysiological studies of olfaction. Behav. Res. Methods Instrum. Comput. 31, 370–375 (1999).
Hubel, D.H. Tungsten microelectrode for recording from single units. Science 125, 549–550 (1957).
Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004).
Sakaguchi, S. et al. Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature 380, 528–531 (1996).
Fischer, M. et al. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15, 1255–1264 (1996).
Radovanovic, I. et al. Truncated prion protein and Doppel are myelinotoxic in the absence of oligodendrocytic PrPC. J. Neurosci. 25, 4879–4888 (2005).
Prinz, M. et al. Intrinsic resistance of oligodendrocytes to prion infection. J. Neurosci. 24, 5974–5981 (2004).
Montrasio, F. et al. B lymphocyte–restricted expression of prion protein does not enable prion replication in prion protein knockout mice. Proc. Natl. Acad. Sci. USA 98, 4034–4037 (2001).
Raeber, A.J. et al. Ectopic expression of prion protein (PrP) in T lymphocytes or hepatocytes of PrP knockout mice is insufficient to sustain prion replication. Proc. Natl. Acad. Sci. USA 96, 3987–3992 (1999).
Genoud, N. et al. Disruption of Doppel prevents neurodegeneration in mice with extensive Prnp deletions. Proc. Natl. Acad. Sci. USA 101, 4198–4203 (2004).
Acknowledgements
The authors thank members of the Aguzzi laboratory for their assistance, especially G. Miele and P. Schwartz. We also thank J. Manson for the use of the Edinburgh Prnp−/− line, D.-J. Zou and D. Kelley for helpful comments on the behavioral experiments, and J. Gordon for valuable discussions on the electrophysiology data. This work was supported by grants from the National Institute on Deafness and Other Communication Disorders (S.F., C.E.L.P., M.T.V., B.T.S. and A.T.C.). C.E.L.P. also received a Short-Term Fellowship from the European Molecular Biology Organization. A.A. and M.P. were supported by grants from the European Community and the Swiss National Science Foundation.
Author information
Authors and Affiliations
Contributions
C.E.L.P., A.T.C., M.P., A.A. and S.F. designed the behavior experiments. M.T.V., B.T.S. and S.F. conceived the electrophysiology experiments. C.E.L.P., A.T.C. and M.P. carried out the cookie-finding behavior experiments and C.E.L.P. and M.T.V. performed the habituation-dishabituation test. C.E.L.P. analyzed all of the behavior experiments and carried out the behavior control experiments. B.T.S. designed the electrophysiology setup. M.T.V. performed the electrophysiology experiments and their analysis. C.E.L.P., M.T.V. and S.F. wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Methods (PDF 4142 kb)
Rights and permissions
About this article
Cite this article
Le Pichon, C., Valley, M., Polymenidou, M. et al. Olfactory behavior and physiology are disrupted in prion protein knockout mice. Nat Neurosci 12, 60–69 (2009). https://doi.org/10.1038/nn.2238
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2238
This article is cited by
-
Mechanisms of prion-induced toxicity
Cell and Tissue Research (2023)
-
Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease
Molecular Neurodegeneration (2020)
-
The function of the cellular prion protein in health and disease
Acta Neuropathologica (2018)
-
Activation of zebrafish Src family kinases by the prion protein is an amyloid-β-sensitive signal that prevents the endocytosis and degradation of E-cadherin/β-catenin complexes in vivo
Molecular Neurodegeneration (2016)
-
Involvement of PrPC in kainate-induced excitotoxicity in several mouse strains
Scientific Reports (2015)