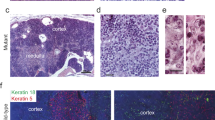
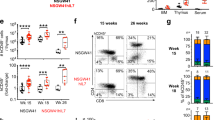
Abstract
Inherited deficiency of the CD40 ligand (X-linked hyper-IgM syndrome) is characterized by failure of immunoglobulin isotype switching and severe defects of cell-mediated immunity. To test the potential for gene transfer therapy to correct this disorder, we transduced murine bone marrow or thymic cells with a retroviral vector containing the cDNA for the murine CD40 ligand (CD40L) and injected them into CD40L –/– mice. Even low-level, constitutive expression of the transgene stimulated humoral and cellular immune functions in these mice. With extended follow-up, however, 12 of 19 treated mice developed T-lymphoproliferative disorders, ranging from polyclonal increases of lymphoblasts to overt monoclonal T-Lymphoblastic lymphomalymphomas that involved multiple organs. Our findings show that constitutive (rather than tightly regulated), low-level expression of CD40L can produce abnormal proliferative responses in developing T lymphocytes, apparently through aberrant interaction between CD40L + and TCRαβ + CD40 + thymocytes. Current methods of gene therapy may prove inappropriate for disorders involving highly regulated genes in essential positions in proliferative cascades.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dunn, R.J., Luedecker, C.J., Haugen, H.S., Clegg, C.H., & Farr, A.G. Thymic overexpression of CD40 ligand disrupts normal thymic epithelial organization. J. Histochem. Cytochem. 45, 129-141 (1997 ).
van Kooten. C. & Banchereau, J. CD40-CD40 ligand: a multifunctional receptor-ligand pair. Adv. Immunol. 61, 1-77 (1996).
Hasbold, J., Johnson-Leger, C., Atkins, C.J., Clark, E.A. & Klaus, G.B. Properties of mouse CD40: cellular distribution of CD40 and B cell activation by monoclonal anti-mouse CD40 antibodies. Eur. J. Immunol. 24, 1835-1842 (1994).
Grewal, I.S. & Flavell, R.A. The CD40 ligand - at the center of the immune universe? Immunol. Res. 16, 59-70 (1997).
Stout, R.D. & Suttles, J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol. Today 17, 487-492 (1996).
Noelle, R.J. CD40 and its ligand in host defence. Immunity 4, 415-419 (1996).
Hollenbaugh, D., Ochs, H.D., Noelle, R.J., Ledbetter, J.A. & Aruffo, A. The role of CD40 and its ligand in the regulation of the immune response. Immunol. Rev. 138, 23-37 (1994).
Clark, E.A. & Ledbetter, J.A. How B and T cells talk to each other. Nature 367, 425-428 (1994).
Kroczek, R.A. et al. Defective expression of CD40 ligand on T cells causes "X-linked immunodeficiency with hyper-IgM (HIGM1)". Immunol. Rev. 138, 39-59 (1994).
Fuleihan, R.L. & Geha, R.S. X-linked hyperIgM. The Immunologist 5, 133-136 (1997).
Malech, H.L. et al. Prolonged production of NADPH oxidase-corrected granulocytes after gene therapy of chronic granulomatous disease. Proc. Natl. Acad. Sci. USA 94, 12133-12138 (1997).
Kohn, D.B. Gene therapy for hematopoietic and immune disorders. Bone Marrow Transplant. 18 (Suppl. 3), S55-S58 (1996).
Renshaw, B.R. et al. Humoral immune responses in CD40 ligand-deficient mice. J. Exp. Med. 180, 1889-1900 ( 1994).
Strzadala, L., Miazek, A., Matuszyk, J. & Kisielow, P. Role of thymic selection in the development of thymic lymphomas in TCR transgenic mice. Int. Immunol. 9, 127-138 ( 1997).
Tokoro, Y., Tsuda, S., Tanaka, S., Nakauchi, H. & Takahama, Y. CD3-induced apoptosis of CD4+ CD8+ thymocytes in the absence of clonotypic T cell antigen receptor. Eur. J. Immunol. 26, 1012-1017 (1996).
Sentman, C.L., Shutter, J.R., Hockenbery, D., Kanagawa, O. & Korsmeyer, S.J. Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 67, 879-888 (1991).
Peguet-Navarro, J. et al. CD40 ligation of human keratinocytes inhibits their proliferation and induces their differentiation. J. Immunol. 158, 144-152 (1997).
Heath, A.W. et al. Antibodies to murine CD40 stimulate normal B lymphocytes but inhibit proliferation of B lymphoma cells. Cell. Immunol. 152, 468-480 (1993).
Funakoshi, S. et al. Inhibition of human B-cell lymphoma growth by CD40 stimulation. Blood 83, 2787-2794 (1994).
Shortman, K., Vremec, D. & Egerton, M. The kinetics of T cell antigen receptor expression by subgroups of CD4+8+ thymocytes: delineation of CD4+8+3(2+) thymocytes as post-selection intermediates leading to mature T cells. J. Exp. Med. 173, 323-332 ( 1991).
Kisielow, P. & von Boehmer H. Development and selection of T cells: facts and puzzles. Adv. Immunol. 58, 87-209 ( 1995).
Bunting, K.D., Sangster, M.Y., Ihle, J.N. & Sorrentino, B.P. Restoration of lymphocyte function in Janus kinase 3-deficient mice by retroviral-mediated gene transfer. Nature Med. 4, 58- 64 (1998).
Einerhand, M.P., Bakx, T.A., Kukler, A. & Valerio, D. Factors affecting the transduction of pluripotent hematopoietic stem cells: long-term expression of a human adenosine deaminase gene in mice. Blood 81, 254-263 (1993).
Qazilbash, M.H. et al. Retroviral vector for gene therapy of X-linked severe combined immunodeficiency syndrome. J.Hematother. 4 , 91-98 (1995).
Clegg, C.H. et al. Thymus dysfunction and chronic inflammatory disease in g39 transgenic mice. Int. Immunol. 9, 1111- 1122 (1997).
Foy, T.M. et al. An essential role for gp39, the ligand for CD40, in thymic selection. J. Exp. Med. 182, 1377- 1388 (1995).
Miller, G. in Virology 2nd edn. (eds. Fields, B.N. et al.) 563– 589 (Raven, New York, 1990).
Cayabyab, M., Phillips, J.H. & Lanier, L.L. CD40 preferentially costimulates activation of CD4+ T lymphocytes. J. Immunol. 152, 1523- 1531 (1994).
Armitage, R.J. et al. CD40 ligand is a potent T cell growth factor. Eur. J. Immunol. 23, 2326-2331 (1993).
Fanslow, W.C. et al. Recombinant CD40 ligand exerts potent biologic effects on T cells. J. Immunol. 152, 4262- 4269 (1994).
Yang, Y. & Wilson, J.M. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science 273, 1862-1867 (1996).
Zhou, T., Bluethmann, H., Eldridge, J., Berry, K. & Mountz, J.D. Origin of CD4–CD8–B220+ T cells in MRL-lpr/lpr mice; clues from a T cell receptor β transgenic mouse. J. Immunol. 150, 3651-3667 (1993).
Zhou, T. et al. Kinetics of Fas-induced apoptosis in thymic organ culture. J. Clin. Immunol. 17, 74-84 ( 1997).
Ezine, S. et al. A novel CD45RA+ CD4+ transient thymic subpopulation in MRL-lpr/lpr mice : its relation to non-proliferating CD4– CD8– CD45RA+ tumor cells. Int. Immunol. 5, 89- 96 (1993).
Smith, G.S., Walford, R.L. & Mickey, R.M. Lifespan and incidence of cancer and other diseases in selected long-lived inbred mice and their F1 hybrids. J. Natl. Cancer Inst. 50, 1195 (1973).
Hoag, W.G. Spontaneous cancer in mice. Ann. N.Y Acad. Sci. 108 , 805 (1963).
Filipovich, A.H. et al. in The Non-Hodgkin's Lymphomas 2nd edn. (ed. McGrath, I.T.) 459-469 (Oxford University Press, New York, 1997).
Larson, R.C. et al. Protein dimerization between Lmo2 (Rbtn2) and Tal1 alters thymocyte development and potentiates T cell tumorigenesis in transgenic mice. EMBO. J. 15, 1021-1027 (1996).
Anderson, W.F. Human gene therapy. Nature 392, 25– 30 (1998).
Grossmann, M.E., Brown, M.P. & Brenner, M.K. Antitumor responses induced by transgenic expression of CD40 ligand. Hum. Gene. Ther. 8, 1935 -1940 (1997).
Farina, S.F., Girard, L.J., Vanin, E.F., Nienhuis, A.W. & Bodine, D.M. Dysregulated expression of GATA-1 following retrovirus-mediated gene transfer into murine hematopoietic stem cells increases erythropoiesis. Blood 86, 4124-4133 (1995).
McLachlin, J.R., Mittereder, N., Daucher, M.B., Kadan, M. & Eglitis, M.A. Factors affecting retroviral vector function and structural integrity. Virology 195, 1-5 (1993).
Hanenberg, H. et al. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nature Med. 2, 876-882 ( 1996).
Heslop, H.E. et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature Med. 2, 551-555 (1996).
Sorrentino, B.P. et al. Selection of drug-resistant bone marrow cells in vivo after retroviral transfer of human MDR1. Science 257, 99-103 (1992).
Allan, W., Tabi, Z., Cleary, A. & Doherty, P.C. Cellular events in the lymph node and lung of mice with influenza. J. Immunol. 144, 3980-3986 (1990).
Flynn, K.J. & Mullbacher, A. Memory alloreactive cytotoxic T cells do not require costimulation for activation in vitro. Immunol. Cell. Biol. 74, 413-420 (1996).
Sangster, M., Smith, F.S., Coleclough, C. & Hurwitz, J.L. Human parainfluenza virus type 1 immunization of infant mice protects from subsequent sendai virus infection. Virology 212, 13-19 (1995).
Harlow, E, & Lane, D. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, 99 ( 1988).
Cole, G.A., Hogg, T.L. & Woodland, D.L. The MHC class I-restricted T cell response to Sendai virus infection in C57BL/6 mice: a single immunodominant epitope elicits an extremely diverse repertoire of T cells. Int. Immunol. 6, 1767-1775 (1994).
Vanin, E.F., Kaloss, M., Broscius, C. & Nienhuis, A.W. Characterization of replication-competent retroviruses from nonhuman primates with virus-induced T-cell lymphomas and observations regarding the mechanism of oncogenesis. J. Virol. 68, 4241-4250 (1994).
Daniels, C.A., Bodner, S. & Trofatter, K.F. Scanning and transmission electron microscopic studies of complement-mediated lysis and antibody-dependent cell-mediated cytolysis of herpes simplex virus-infected human fibroblasts. Am. J. Pathol . 100, 663-675 (1980 ).
Acknowledgements
We thank S. Bodner for electron microscopy; M. Leventhal for immunohistochemistry; J. Gunelson, S. Wingo, A. Slusher and M. Holladay for technical assistance; and J. Gilbert for scientific editing. The work in the authors' laboratories is supported by United States Public Health Service grants AI-29579 to P.C.D.,AI-37597 to D.L.W., CA 78792 and CA 75014 to M.K.B., the Assisi Foundation and the American Lebanese Syrian Associated Charities(ALSAC).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brown, M., Topham, D., Sangster, M. et al. Thymic lymphoproliferative disease after successful correction of CD40 ligand deficiency by gene transfer in mice. Nat Med 4, 1253–1260 (1998). https://doi.org/10.1038/3233
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/3233
This article is cited by
-
Enhancing Antitumor Efficacy of Chimeric Antigen Receptor T Cells Through Constitutive CD40L Expression
Molecular Therapy (2015)
-
Gene Therapy for Primary Immunodeficiencies: Current Status and Future Prospects
Drugs (2014)
-
The Hyper IgM Syndromes
Clinical Reviews in Allergy & Immunology (2014)
-
Gene transfer into hematopoietic stem cells as treatment for primary immunodeficiency diseases
International Journal of Hematology (2014)
-
Spliceosome-Mediated Trans-Splicing: The Therapeutic Cut and Paste
Journal of Investigative Dermatology (2012)