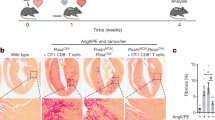
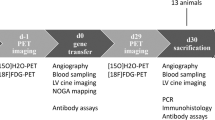
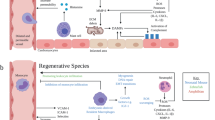
Abstract
The immune response to adenoviral vectors can induce inflammation and loss of transgene expression in transfected tissues. This would limit the use of adenovirus-mediated gene transfer in disease states in which long-term gene expression is required. While studying the effect of the anti-adenoviral immune response in transplantation, we found that transgene expression persisted in cardiac isografts transfected with an adenovirus encoding β-galactosidase. Transfected grafts remained free of inflammation, despite the presence of an immune response to the vector. Thus, adenovirus-mediated gene transfer may have therapeutic value in cardiac transplantation and heart diseases. Furthermore, immunological limitations of adenoviral vectors for gene therapy are not universal for all tissue types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wilson, J.M. Adenoviruses as gene-delivery vehicles. N. Engl. J. Med. 334, 1185–1187 (1996).
Trapnell, B.C. & Gorziglia, M. Gene therapy using adenoviral vectors. Curr. Opin. Biotech. 5, 617– 625 (1994).
Yang, Y. et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA 91, 4407–4411 (1994).
Yang, Y., Li, Q., Ertl, H.C.J. & Wilson, J.M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 69, 2004–2015 (1995).
Tripathy, S.K., Black, H.B., Goldwasser, E. & Leiden, J. M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nature Med. 2, 545–550 ( 1996).
Yang, Y., Jooss, K.U., Su, Q., Ertl, H.C.J. & Wilson, J. M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 3, 137– 144 (1996).
Yang, Y. & Wilson, J. M. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J. Immunol. 155, 2564–2570 (1995).
DeMatteo, R.P., Markmann, J.F., Kozarsky, K.F., Barker, C.F. & Raper, S.E. Prolongation of adenoviral transgene expression in mouse liver by T lymphocyte subset depletion. Gene Ther. 3, 4–12 ( 1996).
Vilquin, J.-T. et al. FK506 immunosuppression to control the immune reactions triggered by first-generation adenovirus-mediated gene transfer. Human Gene Ther. 6, 1391–1401 ( 1995).
Gao, X. & Huang, L. Cationic liposome-mediated gene transfer. Gene Ther. 2, 710–722 (1995).
Crystal, R.G. The gene as the drug. Nature Med. 1, 15– 17 (1995).
Gunzburg, W. H., & Salmons, B. Development of retroviral vectors as safe, targeted gene delivery systems. J. Mol. Med. 74, 171–182 ( 1996).
Larsen, C.P. et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 381, 434–438 (1996).
Bishop, D.K., Shelby, J. & Eichwald, E. J. Mobilization of T lymphocytes after cardiac transplantation: Evidence that CD4-positive cells are required for cytotoxic T lymphocyte activation, inflammatory enothelial development, graft infiltration, and acute allograft rejection. Transplantation 53, 849– 857 (1997).
Merrick, A.F., Shewring, L.D., Sawyer, G.J., Gustafsson, K.T. & Fabre, J.W. Comparison of adenovirus gene transfer to vascular endothelial cells in cell culture, organ culture, and in vivo . Transplantation 62, 1085– 1089 (1996).
Wang, J., Ma, Y. & Knechtle, S.J. Adenovirus-mediated gene transfer into rat cardiac allografts: Comparison of direct injection and perfusion. Transplantation 61, 1726–1729 (1996).
Shiraishi, M. et al. Adenovirus-mediated gene transfer using ex vivo perfusion of the heart graft. Surg. Today 26, 624– 628 (1996).
Lee, J. et al. Cardiac gene transfer by intracoronary infusion of adenovirus vector-mediated reporter gene in the transplanted mouse heart. J. Thorac. Cardiovasc. Surg. 111, 246–252 ( 1996).
Drazan, K.E. et al. Hepatic function is preserved following liver-directed, adenovirus-mediated gene transfer. J. Surg. Res. 59, 299– 304 (1995).
Drazan, K.E. et al. Adenovirus-mediated gene transfer in the transplant setting: Early events after orthotopic transplantation of liver allografts expressing TGF-β1. Transplantation 62, 1080– 1084 (1996).
Shaked, A. et al. Adenovirus-mediated gene transfer in the transplant setting: II. Successful expression of transferred cDNA in syngeneic liver grafts. Transplantation 57, 1508–1511 (1994).
DeMatteo, R.P. et al. Immunologic barriers to hepatic adenoviral gene therapy for transplantation: Cellular and humoral responses limit transgene expression in mouse liver. Transplantation 63, 315– 319 (1997).
McCoy, R.D., Davidson, B.L., Roessler, B.J., Huffnagle, B.B. & Simon, R.H. Expression of human interleukin-1 receptor antagonist in mouse lungs using a recombinant adenovirus: Effects on vector-induced inflammation. Gene Ther. 2, 437–442 (1995).
Qin, L. et al. Adenovirus-mediated gene transfer of viral interleukin-10 inhibits the immune response to both alloantigen and adenoviral antigen. Hum. Gene Ther. 8, 1365–1374 (1997).
Olthoff, K.M. et al. Adenovirus-mediated gene transfer into cold-preserved liver allografts: Survival pattern and unresponsiveness following transduction with CTLA4Ig. Nature Med. 4, 194– 200 (1998).
Austyn, J.M. et al. Isolation and characterization of dendritic cells from mouse heart and kidney. J. Immunol. 152, 2401– 2410 (1994).
Larsen, C.P., Morris, P. J. & Austyn, J.M. Migration of dendritic leukocytes from cardiac allografts into host spleens: A novel pathway for initiation of rejection. J. Exp. Med. 171, 307–314 (1990).
Garside, P. et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281, 96– 99 (1998).
Logan, J. & Shenk, T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc. Natl. Acad. Sci USA 81, 3655–3659 (1984).
Ye, X. et al. Prolonged metabolic correction in adult ornithine transcarbamylase-deficient mice with adenoviral vectors. J. Biol. Chem. 271, 3639–3646 (1996).
Corry, R.J., Winn, H.J. & Russell, P.S. Primarily vascularized allografts of hearts in mice: The role of H-2D, H-2K, and Non-H-2 antigens in rejection. Transplantation 16, 343–350 ( 1973).
Acknowledgements
We thank G. Nabel and M. Imperiale for their comments regarding this manuscript. This work was supported by NIH grant R01 AI HL 31946.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, S., Li, K., Piccotti, J. et al. Tissue-specific consequences of the anti-adenoviral immune response: implications for cardiac transplants. Nat Med 5, 1143–1149 (1999). https://doi.org/10.1038/13467
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/13467