Abstract
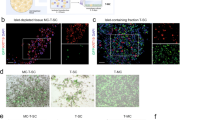
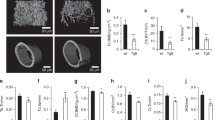
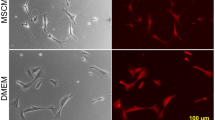
We investigated tetracycline regulation of gene expression in an experimental model relevant to gene therapy. Mouse primary myogenic cells were engineered for doxycycline-inducible and skeletal muscle-specific expression of the mouse erythropoietin (Epo) cDNA by using two retrovirus vectors. Gene expression increased 200 fold in response to both myogenic cell differentiation and doxycycline stimulation. After transplantation of transduced cells into mouse skeletal muscles, Epo secretion could be iteratively switched on and off over a five-month period, depending on the presence or the absence of doxycycline in the drinking water. We conclude that tetracycline regulation provides a suitable control system for adjusting the delivery of therapeutic proteins from engineered tissues over long periods of time.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gossen, M. & Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89, 5547–5551 (1992).
Gossen, M., Bonin, A.L., Freundlieb, S. & Bujard, H. Inducuble gene expression systems for higher eukaryotic cells. Curr. Opin. Biotechnol. 5, 516–520 (1994).
Furth, P.A. et al. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc. Natl. Acad. Sci. U.S.A 91, 9302–9306 (1994).
Shockett, P., Difilippantonio, M., Hellman, N. & Schatz, D. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc. Natl. Acad. Sci. USA 92, 6522–6526 (1995).
Passman, R.S. & Fishman, G.I. Regulated expression of foreign genes in vivo after germline transfer. J. Clin. Invest. 94, 2421–2425 (1994).
Efrat, S., Fusco-DeMane, D., Lemberg, H., Al Emran, O. & Wang, X. Conditional transformation of a pancreatic b-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc. Natl. Acad. Sci. USA 92, 3576–3580 (1995).
Schultze, N., Burki, Y., Lang, Y., Certa, U. & Bluethmann, H. Efficient control of gene expression by single step integration of the tetracycline system in transgenic mice. Nature Biotech. 14, 499–505 (1996).
Gossen, M. et al. Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769 (1995).
Paulus, W., Baur, I., Boyce, P.M., Breakefield, X.O. & Reeves, S.A. Self-contained, tetracycline-regulated retroviral system for gene delivery to mammalian cells. J. Virol. 70, 62–67 (1996).
lida, A., Chen, S.T., Friedmann, T. & Yee, J.K. Inducible gene expression by retro-virus-mediated transfer of a modified tetracycline-regulated system. J. Virol. 70, 6054–6059 (1996).
Hwang, J.J., Scuric, Z. & Anderson, W.F. Novel retroviral vector transferring a suicide gene and a selectable marker gene with enhanced gene expression by using a tetracycline-responsive expression system. J. Virol. 70, 8138–8141 (1996).
Hoshimaru, M., Ray, J., San, D.W.Y. & Cage, F.H. Differentiation of the immortalized adult neuronal progenitor cell line HC2S2 into neurons by regulatable suppression of the v-myc oncogene. Proc. Natl. Acad. Sci. USA 93, 1518–1523 (1996).
Hofman, A., Nolan, G. & Blau, H.B. Rapid retroviral delivery of tetracycline-inducible genes in a single autoregulatory cassette. Proc. Natl. Acad. Sci. USA 93, 5185–5190 (1996).
Li, Z. & Paulin, D. High level desmin expression depends on a muscle-specific enhancer. J. Biol. Chem. 266, 6562–6570 (1991).
Naffakh, N. et al. Long-term secretion of therapeutic proteins from genetically-modified skeletal muscles. Human Gene Ther. 7, 11–21 (1996).
Danos, O. & Mulligan, R.C. Safe and efficient generation of recombinant retro-viruses with amphotropic and ecotropic host ranges. Proc. Natl. Acad. Sci. USA 85, 6460–6464 (1988).
Cosset, F.L., Takeuchi, Y., Battini, J.L., Weiss, R.A. & Collins, M. K. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 69, 7430–7436 (1995).
Cone, R.D., Weber-Benarous, A., Baorto, D. & Mulligan, R.C. Regulated expression of a complete human β-globin gene encoded by a transmissible retrovirus vector. Mol. Cell. Biol. 7, 887–897 (1987).
Guild, B.C., Finer, M.H., Housman, D.E. & Mulligan, R.C. Development of retrovirus vectors useful for expressing genes in cultured murine embryonal cells and hematopoietic cells in vivo. J. Virol. 62, 3795–3801 (1988).
Soriano, P., Friedrich, G. & Lawinger, P. Promoter interactions in retrovirus vectors introduced into fibroblasts and embryonic stem cells. J. Virol. 65, 2314–2319 (1991).
Emerman, M. & Temin, H.M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell 39, 459–467 (1984).
Emerman, M. & Temin, H.M. Quantitative analysis of gene suppression in integrated retrovirus vectors. Mol. Cell. Biol. 6, 792–800 (1986).
Bauer, T.R. Jr., Osborne, W.R.A., Kwok, W.W. & Hickstein, D.D. Expression from leukocyte integrin promoters in retroviral vectors. Human Gene Ther. 5, 709–716 (1994).
Vile, R., Miller, N., Chernajovsky, Y. & Hart, I. A comparison of the properties of different retroviral vectors containing the murine tyrosinase promoter to achieve transcriptionally targeted expression of the HSVtk or IL2 genes. Gene Ther. 1, 307–316 (1994).
Yee, J.K. et al. Gene expression from transcriptionally disabled retroviral vectors. Proc. Natl. Acad. Sci. USA 84, 5199–5201 (1987).
Yu, S. et al. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc. Natl. Acad. Sci. USA 83, 3194–3198 (1986).
Soriano, P., Cone, R.D., Mulligan, R.C. & Jaenisch, R. Tissue-specific and ectopic expression of genes introduced into transgenic mice by retroviruses. Science 234, 1409–1413 (1986).
Jaenisch, R. et al. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell 24, 519–529 (1981).
Hoeben, R.C., Migchielsen, A.A., van der Jagt, R.C.M., van Ormondt, H. & van der Erb, A.J. Inactivation of the Moloney murine leukemia virus long terminal repeat in murine fibroblast cell lines is associated with methylation and dependent on its chromosomal position. J. Virol. 65, 904–912 (1991).
Metcalf, D. The molecular control of blood Cells (Harvard Univ. Press, Cambridge, Massachussets, 1988).
Salmonson, T., Danielson, B.G. & Wikstrom, B. The pharmacokinetics of recombinant erythropoietin after intravenous and subcutaneous administration to healthy subjects. Br. J. Pharmacol. 29, 703–713 (1990).
Jensen, J.D., Jensen, L.W. & Madsen, J.K. The pharmacokinetics of recombinant human erythropoietin after subcutaneous injection at different sites. Eur. J. Clin. Pharmacol. 46, 333–337 (1994).
Klein, J. Cellular and subcellular distribution of H-2 antigens. in Biology of the mouse histocompatibility complex (Springer Verlag, Berlin, pp 329–383. 1975).
Madan, A. & Curtin, P.T. A 24-base-pair sequence 3′ to the human erythropoietin gene contains a hypoxia-responsive transcriptionat enhancer. Proc. Natl. Acad. Sci. USA 90, 3928–3932 (1993).
Madan, A., Lin, C., Hatch II, S.L. & Curtin, P. T. Regulated basal, inducible, and tissue-specific human erythropoietin gene expression in transgenic mice requires multiple cis DMA sequences. Blood 85, 2735–2741 (1995).
Beck, I., Weinmann, R. & Caro, J. Characterization of hypoxia-responsive enhancer in the human erythropoietin gene shows presence of hypoxia-inducible 120-Kd nuclear DNA-binding protein in erythropoietin-producing and nonproducing cells. Blood 82, 704–711 (1993).
Wang, G.L. & Semenza, G.L. General involvment of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA 90, 4304–4308 (1993).
Naffakh, N. et al. Sustained delivery of erythropoietin in mice by autologous implants of genetically-modified skin fibroblasts. Proc. Natl. Acad. Sci. USA 92, 3194–3198 (1995).
Sakaguchi, M. et al. The expression of functional erythropoietin receptors on an interleukin-3 dependent cell line. Biochem. Biophys. Res. Commun. 146, 7 (1987).
Ferry, N., Duplessis, O., Houssin, D., Danos, O. & Heard, J.M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 88, 8377–8381 (1991).
Wright, W.E., Sasoon, D.A. & Lin, V.K., Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 56, 607–617 (1989).
Jacqué, J.M. et al. Permanent occupancy of the human immunodeficiency virus type 1 enhancer by NF-κB is needed for persistent viral replication in monocytes. J. Virol. 70, 2930–2938 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bohl, D., Naffakh, N. & Heard, J. Long-term control of erythropoietin secretion by doxycycline in mice transplanted with engineered primary myoblasts. Nat Med 3, 299–305 (1997). https://doi.org/10.1038/nm0397-299
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0397-299
This article is cited by
-
Erythropoietin gene doping: facts and fictions
Sportwissenschaft (2012)
-
AAV9-mediated erythropoietin gene delivery into the brain protects nigral dopaminergic neurons in a rat model of Parkinson's disease
Gene Therapy (2010)
-
Adeno-associated viral vector-mediated hypoxia-regulated VEGF gene transfer promotes angiogenesis following focal cerebral ischemia in mice
Gene Therapy (2008)
-
Sensitive and precise regulation of haemoglobin after gene transfer of erythropoietin to muscle tissue using electroporation
Gene Therapy (2007)
-
Recombinant AAV-mediated gene transfer to the retina: gene therapy perspectives
Gene Therapy (2004)