Abstract
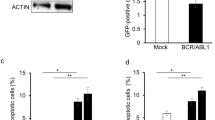
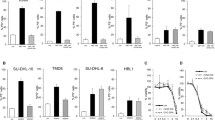
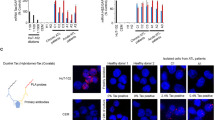
The chimeric BCR–ABL oncoprotein is the molecular hallmark of chronic myelogenous leukemia (CML). BCR–ABL contains nuclear import and export signals but it is localized only in the cytoplasm where it activates mitogenic and anti-apoptotic pathways. We have found that inhibition of the BCR–ABL tyrosine kinase, either by mutation or by the drug STI571, can stimulate its nuclear entry. By combining STI571 with leptomycin B (LMB) to block nuclear export, we trapped BCR–ABL in the nucleus and the nuclear BCR–ABL tyrosine kinase activates apoptosis. As a result, the combined treatment with STI571 and LMB causes the irreversible and complete killing of BCR–ABL transformed cells, whereas the effect of either drug alone is fully reversible. The combined treatment with STI571 and LMB also preferentially eliminates mouse bone marrow cells that express BCR–ABL. These results indicate that nuclear entrapment of BCR–ABL can be used as a therapeutic strategy to selectively kill chronic myelogenous leukemia cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Faderl, S. et al. The biology of chronic myeloid leukemia. N. Engl. J. Med. 341, 164–172 (1999).
Faderl, S., Talpaz, M., Estrov, Z. & Kantarjian, H.M. Chronic myelogenous leukemia: biology and therapy. Ann. Intern. Med. 131, 207–219 (1999).
Warmuth, M., Danhauser-Riedl, S. & Hallek, M. Molecular pathogenesis of chronic myeloid leukemia: implications for new therapeutic strategies. Ann. Hematol. 78, 49–64 (1999).
Daley, G.Q., Van Etten, R.A. & Baltimore, D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 247, 824–830 (1990).
Pear, W.S. et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 92, 3780–3792 (1998).
Zhang, X. & Ren, R. Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice: a novel model for chronic myelogenous leukemia. Blood 92, 3829–3840 (1998).
Wang, J.Y.J. in Integrative signaling through Abl: a tyrosine kinase with nuclear and cytoplasmic functions, 303–324 (Humana Press, Totowa, New Jersey, 2000).
Daley, G.Q., Van Etten, R.A., Jackson, P.K., Bernards, A. & Baltimore, D. Nonmyristoylated Abl proteins transform a factor-dependent hematopoietic cell line. Mol. Cell. Biol. 12, 1864–1871 (1992).
Renshaw, M.W., McWhirter, J.R. & Wang, J.Y. The human leukemia oncogene bcr-abl abrogates the anchorage requirement but not the growth factor requirement for proliferation. Mol. Cell. Biol. 15, 1286–1293. (1995).
Skorski, T. et al. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J. 16, 6151–6161 (1997).
Amarante-Mendes, G.P. et al. Bcr–Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome C and activation of caspase-3. Blood 91, 1700–1705 (1998).
McWhirter, J.R. & Wang, J.Y. An actin-binding function contributes to transformation by the Bcr–Abl oncoprotein of Philadelphia chromosome+ human leukemias. EMBO J. 12, 1533–1546 (1993).
Wen, S.T., Jackson, P.K. & Van Etten, R.A. The cytostatic function of Abl is controlled by multiple nuclear localization signals and requires the p53 and Rb tumor suppressor gene products. EMBO J. 15, 1583–1595 (1996).
Taagepera, S. et al. Nuclear-cytoplasmic shuttling of ABL tyrosine kinase. Proc. Natl. Acad. Sci. USA 95, 7457–7462 (1998).
Fornerod, M., Ohno, M., Yoshida, M. & Mattaj, I.W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90, 1051–1060 (1997).
Fukuda, M. et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390, 308–311 (1997).
Baskaran, R. et al. Ataxia telangiectasia mutant protein activates Abl tyrosine kinase in response to ionizing radiation. Nature 387, 516–519 (1997).
Jost, C.A., Marin, M.C. & Kaelin, W.G., Jr. p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature 389, 191–194 (1997); erratum: 399, 817 (1999).
Gong, J.G. et al. The tyrosine kinase Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 399, 806–809 (1999).
Wang, J.Y.J. Regulation of cell death by the Abl tyrosine kinase. Oncogene 20, 5643–5650 (2000).
Buchdunger, E. et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 56, 100–104 (1996).
Druker, B.J. et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nature Med. 2, 561–566 (1996).
le Coutre, P. et al. In vivo eradication of human BCR/ABL-positive leukemia cells with an ABL kinase inhibitor. J. Natl. Cancer Inst. 91, 163–168 (1999).
Deininger, M.W., Goldman, J.M., Lydon, N. & Melo, J.V. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of Bcr-Abl-positive cells. Blood 90, 3691–3698 (1997).
Beran, M. et al. Selective inhibition of cell proliferation and BCR-ABL phosphorylation in acute lymphoblastic leukemia cells expressing Mr 190,000 BCR-ABL protein by a tyrosine kinase inhibitor (CGP-57148). Clin. Cancer Res. 4, 1661–1672 (1998).
McWhirter, J.R., Galasso, D.L. & Wang, J.Y. A coiled-coil oligomerization domain of Bcr is essential for the transforming function of Bcr–Abl oncoproteins. Mol. Cell. Biol. 13, 7587–7595 (1993).
Schindler, T. et al. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 289, 1938–1942 (2000).
Klein, E. et al. Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int. J. Cancer 18, 421–431 (1976).
Klucher, K.M., Lopez, D.V. & Daley, G.Q. Secondary mutation maintains the transformed state in BaF3 cells with inducible BCR/ABL expression. Blood 91, 3927–3934 (1998).
Kudo, N. et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 96, 9112–9117 (1999).
Li, S., Ilaria, R.L. Jr, Million, R.P., Daley, G.Q. & Van Etten, R.A. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J. Exp. Med. 189, 1399–1412 (1999).
Heinrich, M.C. et al. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood 96, 925–932 (2000).
McGahon, A. et al. BCR-ABL maintains resistance of chronic myelogenous leukemia cells to apoptotic cell death. Blood 83, 1179–1187 (1994).
Druker, B.J. & Lydon, N.B. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J. Clin. Invest. 105, 3–7 (2000).
Goldman, J.M. Tyrosine-kinase inhibition in treatment of chronic myeloid leukaemia. Lancet 355, 1031–1032 (2000).
Vastag, B. Leukemia drug heralds molecularly targeted era. J. Natl. Cancer Inst. 92, 6–8 (2000).
le Coutre, P. et al. Induction of resistance to the Abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood 95, 1758–1766 (2000).
Weisberg, E. & Griffin, J.D. Mechanism of resistance to the ABL tyrosine kinase inhibitor STI571 in BCR/ABL-transformed hematopoietic cell lines. Blood 95, 3498–3505 (2000).
Mahon, F.X. et al. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood 96, 1070–1079 (2000).
Gambacorti-Passerini, C. et al. Role of alpha1 Acid Glycoprotein in the In Vivo Resistance of Human BCR-ABL(+) Leukemic Cells to the Abl Inhibitor STI571. J. Natl. Cancer Inst. 92, 1641–1650 (2000).
Newlands, E.S., Rustin, G.J. & Brampton, M.H. Phase I trial of elactocin. Br. J. Cancer 74, 648–649 (1996).
McWhirter, J.R. & Wang, J.Y. Activation of tyrosinase kinase and microfilament-binding functions of Abl by bcr sequences in bcr/abl fusion proteins. Mol. Cell. Biol. 11, 1553–1565. (1991).
Cortez, D., Kadlec, L. & Pendergast, A.M. Structural and signaling requirements for BCR-ABL-mediated transformation and inhibition of apoptosis. Mol. Cell. Biol. 15, 5531–5541 (1995).
Pear, W.S., Nolan, G.P., Scott, M.L. & Baltimore, D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90, 8392–8396 (1993).
Acknowledgements
We thank B. Druker for providing STI571; M. Yoshida for his gift of LMB; G. Daley for the TonB cell line; R. Ren for the MSCV-BCR–ABL/p210-IRES-GFP construct; and J. Feramisco and B. Smith for their assistance with deconvolution microscopy. This work was supported by NIH grants CA 43054 and HL57900 to JYJW, and a fellowship from the Lopiccola Foundation to PV.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vigneri, P., Wang, J. Induction of apoptosis in chronic myelogenous leukemia cells through nuclear entrapment of BCR–ABL tyrosine kinase. Nat Med 7, 228–234 (2001). https://doi.org/10.1038/84683
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/84683
This article is cited by
-
Selective inhibition of nuclear export: a promising approach in the shifting treatment paradigms for hematological neoplasms
Leukemia (2022)
-
Transport and metabolism of tyrosine kinase inhibitors associated with chronic myeloid leukemia therapy: a review
Molecular and Cellular Biochemistry (2022)
-
Effect of HSP90AB1 and CC domain interaction on Bcr-Abl protein cytoplasm localization and function in chronic myeloid leukemia cells
Cell Communication and Signaling (2021)
-
The nuclear export protein XPO1 — from biology to targeted therapy
Nature Reviews Clinical Oncology (2021)
-
Targeting nuclear import and export in hematological malignancies
Leukemia (2020)