Abstract
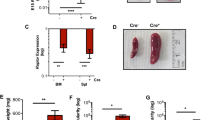
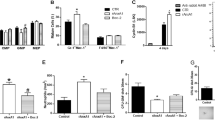
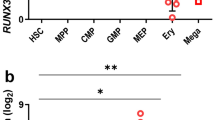
Erythropoietin (EPO) stimulates proliferation of early-stage erythrocyte precursors and is widely used for the treatment of chronic anemia. However, several types of EPO-resistant anemia are characterized by defects in late-stage erythropoiesis1,2, which is EPO independent2,3. Here we investigated regulation of erythropoiesis using a ligand-trapping fusion protein (ACE-536) containing the extracellular domain of human activin receptor type IIB (ActRIIB) modified to reduce activin binding. ACE-536, or its mouse version RAP-536, produced rapid and robust increases in erythrocyte numbers in multiple species under basal conditions and reduced or prevented anemia in murine models. Unlike EPO, RAP-536 promoted maturation of late-stage erythroid precursors in vivo. Cotreatment with ACE-536 and EPO produced a synergistic erythropoietic response. ACE-536 bound growth differentiation factor-11 (GDF11) and potently inhibited GDF11-mediated Smad2/3 signaling. GDF11 inhibited erythroid maturation in mice in vivo and ex vivo. Expression of GDF11 and ActRIIB in erythroid precursors decreased progressively with maturation, suggesting an inhibitory role for GDF11 in late-stage erythroid differentiation. RAP-536 treatment also reduced Smad2/3 activation, anemia, erythroid hyperplasia and ineffective erythropoiesis in a mouse model of myelodysplastic syndromes (MDS). These findings implicate transforming growth factor-β (TGF-β) superfamily signaling in erythroid maturation and identify ACE-536 as a new potential treatment for anemia, including that caused by ineffective erythropoiesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bowen, D. What is ineffective erythropoiesis in myelodysplastic syndromes? Leuk. Lymphoma 18, 243–247 (1995).
Libani, I.V. et al. Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in β-thalassemia. Blood 112, 875–885 (2008).
Eshghi, S., Vogelezang, M.G., Hynes, R.O., Griffith, L.G. & Lodish, H.F. α4β1 integrin and erythropoietin mediate temporally distinct steps in erythropoiesis: integrins in red cell development. J. Cell Biol. 177, 871–880 (2007).
Smith, R.E. Jr. The clinical and economic burden of anemia. Am. J. Manag. Care 16 (suppl.), S59–S66 (2010).
Hattangadi, S.M., Wong, P., Zhang, L., Flygare, J. & Lodish, H.F. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood 118, 6258–6268 (2011).
Tanno, T. & Miller, J.L. Iron loading and overloading due to ineffective erythropoiesis. Adv. Hematol. 2010, 358283 (2010).
Ginzburg, Y. & Rivella, S. β-thalassemia: a model for elucidating the dynamic regulation of ineffective erythropoiesis and iron metabolism. Blood 118, 4321–4330 (2011).
Cao, A., Moi, P. & Galanello, R. Recent advances in β-thalassemias. Pediatr. Rep. 3, e17 (2011).
Jabbour, E., Kantarjian, H.M., Koller, C. & Taher, A. Red blood cell transfusions and iron overload in the treatment of patients with myelodysplastic syndromes. Cancer 112, 1089–1095 (2008).
Rider, C.C. & Mulloy, B. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem. J. 429, 1–12 (2010).
Yu, J. et al. Importance of FSH-releasing protein and inhibin in erythrodifferentiation. Nature 330, 765–767 (1987).
Broxmeyer, H.E. et al. Selective and indirect modulation of human multipotential and erythroid hematopoietic progenitor cell proliferation by recombinant human activin and inhibin. Proc. Natl. Acad. Sci. USA 85, 9052–9056 (1988).
Shiozaki, M., Kosaka, M. & Eto, Y. Activin A: a commitment factor in erythroid differentiation. Biochem. Biophys. Res. Commun. 242, 631–635 (1998).
Maguer-Satta, V. et al. Regulation of human erythropoiesis by activin A, BMP2, and BMP4, members of the TGFβ family. Exp. Cell Res. 282, 110–120 (2003).
Akel, S., Petrow-Sadowski, C., Laughlin, M.J. & Ruscetti, F.W. Neutralization of autocrine transforming growth factor-β in human cord blood CD34+CD38−Lin− cells promotes stem-cell-factor-mediated erythropoietin-independent early erythroid progenitor development and reduces terminal differentiation. Stem Cells 21, 557–567 (2003).
Söderberg, S.S., Karlsson, G. & Karlsson, S. Complex and context dependent regulation of hematopoiesis by TGF-β superfamily signaling. Ann. NY Acad. Sci. 1176, 55–69 (2009).
Shav-Tal, Y. & Zipori, D. The role of activin A in regulation of hemopoiesis. Stem Cells 20, 493–500 (2002).
Blank, U. & Karlsson, S. The role of Smad signaling in hematopoiesis and translational hematology. Leukemia 25, 1379–1388 (2011).
Shiozaki, M. et al. Evidence for the participation of endogenous activin A/erythroid differentiation factor in the regulation of erythropoiesis. Proc. Natl. Acad. Sci. USA 89, 1553–1556 (1992).
Zhou, L. et al. Inhibition of the TGF-β receptor I kinase promotes hematopoiesis in MDS. Blood 112, 3434–3443 (2008).
Cadena, S.M. et al. Administration of a soluble activin type IIB receptor promotes skeletal muscle growth independent of fiber type. J. Appl. Physiol. 109, 635–642 (2010).
Suragani, R.N. et al. Heme-regulated eIF2α kinase activated Atf4 signaling pathway in oxidative stress and erythropoiesis. Blood 119, 5276–5284 (2012).
Socolovsky, M. et al. Ineffective erythropoiesis in Stat5a−/−5b−/− mice due to decreased survival of early erythroblasts. Blood 98, 3261–3273 (2001).
Liu, Y. et al. Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood 108, 123–133 (2006).
Chen, K. et al. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc. Natl. Acad. Sci. USA 106, 17413–17418 (2009).
Camaschella, C. BMP6 orchestrates iron metabolism. Nat. Genet. 41, 386–388 (2009).
McPherron, A.C., Lawler, A.M. & Lee, S.J. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat. Genet. 22, 260–264 (1999).
Wu, H.H. et al. Autoregulation of neurogenesis by GDF11. Neuron 37, 197–207 (2003).
Kim, J. et al. GDF11 controls the timing of progenitor cell competence in developing retina. Science 308, 1927–1930 (2005).
Loffredo, F.S. et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839 (2013).
Lin, Y.W., Slape, C., Zhang, Z. & Aplan, P.D. NUP98-HOXD13 transgenic mice develop a highly penetrant, severe myelodysplastic syndrome that progresses to acute leukemia. Blood 106, 287–295 (2005).
Paulson, R.F., Shi, L. & Wu, D.C. Stress erythropoiesis: new signals and new stress progenitor cells. Curr. Opin. Hematol. 18, 139–145 (2011).
Iancu-Rubin, C. et al. Stromal cell-mediated inhibition of erythropoiesis can be attenuated by Sotatercept (ACE-011), an activin receptor type II ligand trap. Exp. Hematol. 41, 155–166.e17 (2013).
Sherman, M.L. et al. Multiple-dose, safety, pharmacokinetic, and pharmacodynamic study of sotatercept (ActRIIA-IgG1), a novel erythropoietic agent, in healthy postmenopausal women. J. Clin. Pharmacol. 53, 1121–1130 (2013).
Sako, D. et al. Characterization of the ligand binding functionality of the extracellular domain of activin receptor type IIb. J. Biol. Chem. 285, 21037–21048 (2010).
Lee, H.B. & Blaufox, M.D. Blood volume in the rat. J. Nucl. Med. 26, 72–76 (1985).
Lau, A.L., Kumar, T.R., Nishimori, K., Bonadio, J. & Matzuk, M.M. Activin βC and βE genes are not essential for mouse liver growth, differentiation, and regeneration. Mol. Cell. Biol. 20, 6127–6137 (2000).
Menon, M.P. et al. Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J. Clin. Invest. 116, 683–694 (2006).
Ignotz, R.A. & Honeyman, T. TGF-β signaling in A549 lung carcinoma cells: lipid second messengers. J. Cell. Biochem. 78, 588–594 (2000).
Scharpfenecker, M. et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J. Cell Sci. 120, 964–972 (2007).
Townson, S.A. et al. Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. J. Biol. Chem. 287, 27313–27325 (2012).
Roy, C.N. et al. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood 109, 4038–4044 (2007).
Koulnis, M. et al. Identification and analysis of mouse erythroid progenitors using the CD71/TER119 flow-cytometric assay. J. Vis. Exp. 54, –e2809 (2011).
Acknowledgements
We thank J. Knopf for critical review and T. Ahern for editorial contributions. We acknowledge the Cell Biology, Protein Purification and Preclinical Pharmacology groups at Acceleron Pharma for their contributions in support of this work. We also thank G. Paradis and A. Parmelee for their assistance with flow cytometry. NUP98-HOXD13 MDS mice and age-matched FVB wild-type mice were obtained from P. Aplan's laboratory at the US National Institutes of Health. Inhbctm1Zuk and Inhbctm2Zuk mice were obtained from M. Matzuk, Baylor College of Medicine.
Author information
Authors and Affiliations
Contributions
R.N.V.S.S., S.M. Cadena, S.M. Cawley, J.D.Q., R.C., R.S.P., J.S. and R.K. planned and designed the experiments. R.N.V.S.S., S.M. Cadena, S.M. Cawley, D.S. and R.L. conducted the experiments. R.N.V.S.S., S.M. Cadena, S.M. Cawley, D.S., D.M., R.L., M.V.D., M.D., K.S.L., K.W.U. and A.V.G. collected and interpreted data. R.N.V.S.S. and M.J.A. drafted and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.N.V.S.S., S.M. Cadena, S.M. Cawley, D.S., D.M., R.L., M.V.D., M.J.A., K.S.L., K.W.U., A.V.G., J.D.Q., R.S.P., J.S. and R.K. are current or former employees of Acceleron Pharma with ownership interest in the company. R.C. is an employee of Celgene with ownership interest in the company.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 and Supplementary Tables 1–6 (PDF 22824 kb)
Rights and permissions
About this article
Cite this article
Suragani, R., Cadena, S., Cawley, S. et al. Transforming growth factor-β superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat Med 20, 408–414 (2014). https://doi.org/10.1038/nm.3512
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3512
This article is cited by
-
The evolution of preclinical models for myelodysplastic neoplasms
Leukemia (2024)
-
Role of innate immunological/inflammatory pathways in myelodysplastic syndromes and AML: a narrative review
Experimental Hematology & Oncology (2023)
-
Comprehensive characterization of TGFB1 across hematological malignancies
Scientific Reports (2023)
-
Context-dependent TGFβ family signalling in cell fate regulation
Nature Reviews Molecular Cell Biology (2023)
-
TMPRSS6 as a Therapeutic Target for Disorders of Erythropoiesis and Iron Homeostasis
Advances in Therapy (2023)