Abstract
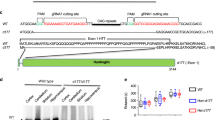
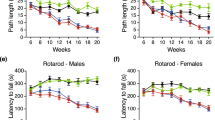
Huntington disease (HD) is an adult-onset, autosomal dominant inherited human neurodegenerative disorder characterized by hyperkinetic involuntary movements, including motor restlessness and chorea, slowing of voluntary movements and cognitive impairment. Selective regional neuron loss and gliosis in striatum, cerebral cortex, thalamus, subthalamus and hippocampus 1,2,3,4 are well recognized as neuropathological correlates for the clinical manifestations of HD. The underlying genetic mutation is the expansion of CAG trinucleotide repeats (coding for polyglutamines) to 36-121 copies in exon 1 of the HD gene 5,6,7,8. The HD mRNA and protein product (huntingtin) show widespread distribution9,10,11, and thus much remains to be understood about the selective and progressive neurodegeneration in HD. To create an experimental animal model for HD, transgenic mice were generated showing widespread expression of full-length human HD cDNA with either 16, 48 or 89 CAG repeats. Only mice with 48 or 89 CAG repeats manifested progressive behavioural and motor dysfunction with neuron loss and gliosis in striatum, cerebral cortex, thalamus and hippocampus. These animals represent clinically relevant models for HD pathogenesis, and may provide insights into the underlying pathophysiological mechanisms of other triplet repeat disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vonsattel, J.P. et al. Neuropathological classification of Huntington's disease. J. Neuropathol. Exp. Neurol. 44, 559– 577 (1985).
Folstein, S.E. Huntington's Disease (Johns Hopkins University Press, Baltimore, 1990).
Hedreen, J.C., Peyser, C.E., Folstein, S.E. & Ross, C.A. Neuronal loss in layers V and VI of cerebral cortex in Huntington's disease. Neurosci. Lett. 133, 257– 261 (1991).
Spargo, E., Everall, I.P. & Lantos, P.L. Neuronal loss in the hippocampus in Huntington's disease: a comparison with HIV infection. J. Neurol. Neurosurg. Psychiatry 56, 487–491 ( 1993).
The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993).
Andrew, S.E. et al. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nature Genet. 4, 398–403 (1993).
Duyao, M. et al. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nature Genet. 4, 387– 392 (1993).
Stine, O.C. et al. Correlation between the onset age of Huntington's disease and length of the trinucleotide repeat in IT-15. Hum. Mol. Genet . 2, 1547–1549 ( 1993).
Li, S.H. et al. Huntington's disease gene (IT15) is widely expressed in human and rat tissues. Neuron 11, 985– 993 (1993).
Strong, T.V. et al. Widespread expression of the human and rat Huntington's disease gene in brain and nonneural tissues. Nature Genet. 5, 259–265 (1993).
Sharp, A.H. et al. Widespread expression of Huntington's disease gene (IT15) protein product. Neuron 14, 1065– 1074 (1995).
Mangiarini, L. et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506 ( 1996).
Ordway, J.M. et al. Ectopically expressed CAG repeats cause intranuclear inclusions and a progressive late onset neurological phenotype in the mouse. Cell 91, 753–764 ( 1997).
Wood, J.D., MacMillan, J.C., Harper, P.S., Lowenstein, P.R. & Jones, A.L. Partial characterisation of murine huntingtin and apparent variations in the subcellular localisation of huntingtin in human, mouse and rat brain. Hum. Mol. Genet. 5, 481–487 (1996).
White, J.K. et al. Huntingtin is required for neurogenesis and is not impaired by the Huntington's disease CAG expansion. Nature Genet. 17, 404–410 (1997).
Franklin, K.B.J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates (Academic Press, San Diego, 1997).
Dragunow, M. et al. In situ evidence for DNA fragmentation in Huntington's disease striatum and Alzheimer's disease temporal lobes. Neuroreport 6, 1053–1057 ( 1995).
Portera-Cailliau, C., Hedreen, J.C., Price, D.L. & Koliatsos, K.E. Evidence for apoptotic cell death in Huntington disease and excitotoxic animal models. J. Neurosci. 15, 3775– 3787 (1995).
Thomas, L.B. et al. DNA end labeling (TUNEL) in Huntington's disease and other neuropathological conditions. Exp. Neurol. 133, 265–272 (1995).
Davies, S.W. et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548 ( 1997).
DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
Paulson, H.L. et al. Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron 19, 333–344 (1997).
Skinner, P.J. et al. Ataxin-1 with an expanded glutamine tract alters nuclear matrix-associated structures. Nature 389, 971–974 (1997).
Borlongan, C.V., Koutouzis, T.K. & Sanberg, P.R. 3-Nitropropionic acid animal model and Huntington's disease. Neurosci. Biobehav. Rev. 21, 289 –293 (1997).
Brinkman, R.R., Mezei, M.M., Theilmann, J., Almqvist, E. & Hayden, M.R. The likelihood of being affected with Huntington disease by a particular age, for a specific CAG size. Am. J. Hum. Genet. 60, 1202–1210 (1997).
Kosinski, C.M. et al. Huntingtin immunoreactivity in the rat neostriatum: differential accumulation in projection and interneurons. Exp. Neurol. 144, 239–247 (1997).
Reddy, P.S. & Housman, D.E. The complex pathology of trinucleotide repeats. Curr. Opin. Cell Biol. 9, 364– 372 (1997).
Matilla, A. et al. The cerebellar leucine-rich acidic nuclear protein interacts with ataxin-1. Nature 389, 974– 978 (1997).
Jou, Y.S. & Myers, R.M. Evidence from antibody studies that the CAG repeat in the Huntington disease gene is expressed in the protein. Hum. Mol. Genet. 4, 465– 469 (1995).
Liu, Y.F., Deth, R.C. & Devys, D.E. SH3 domain-dependent association of huntingtin with epidermal growth factor receptor signaling complexes. J. Biol. Chem. 272, 8121–8124 (1997).
Acknowledgements
We thank R. Albin for stimulating discussions and G. Auburger and F.S. Collins for critical reading of the manuscript. We are grateful to M. MacDonald, Y.-F. Liu and R. Myers for generous gifts of HF1, 437 and αHD1 antibodies, respectively. We thank M. Erdos, T. Hernandez, A. Chen, C. Rivas, E. Stockburger and T. Moss for technical assistance. This work was supported in part by USPHS grant NS-28236 to W.O.W.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, P., Williams, M., Charles, V. et al. Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nat Genet 20, 198–202 (1998). https://doi.org/10.1038/2510
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/2510
This article is cited by
-
Contribution of Glial Cells to Polyglutamine Diseases: Observations from Patients and Mouse Models
Neurotherapeutics (2023)
-
Huntington’s disease brain-derived small RNAs recapitulate associated neuropathology in mice
Acta Neuropathologica (2021)
-
Mitochondrial Abnormalities and Synaptic Damage in Huntington’s Disease: a Focus on Defective Mitophagy and Mitochondria-Targeted Therapeutics
Molecular Neurobiology (2021)
-
Antagonistic pleiotropy in mice carrying a CAG repeat expansion in the range causing Huntington’s disease
Scientific Reports (2019)
-
Protective Effects of Antioxidants in Huntington’s Disease: an Extensive Review
Neurotoxicity Research (2019)