Abstract
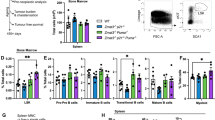
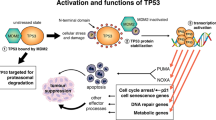
The gene Trp53 is among the most frequently mutated and studied genes in human cancer, but the mechanisms by which it suppresses tumour formation remain unclear. We generated mice with an allele encoding changes at Leu25 and Trp26, known to be essential for transcriptional transactivation and Mdm2 binding, to enable analyses of Trp53 structure and function in vivo. The mutant Trp53 was abundant, its level was not affected by DNA damage and it bound DNA constitutively; however, it showed defects in cell-cycle regulation and apoptosis. Both mutant and Trp53-null mouse embryonic fibroblasts (MEFs) were readily transformed by oncogenes, and the corresponding mice were prone to tumours. We conclude that the determining pathway for Trp53 tumour-suppressor function in mice requires the transactivation domain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ko, L.J. & Prives, C. p53: puzzle and paradigm. Genes Dev. 10, 1054–1072 (1996).
Giaccia, A.J. & Kastan, M.B. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 12, 2973–2983 (1998).
Attardi, L.D., Lowe, S.W., Brugarolas, J. & Jacks, T. Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J. 15, 3693–3701 (1996).
Haupt, Y., Rowan, S., Shaulian, E., Vousden, K.H. & Oren, M. Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev. 9, 2170–2183 (1995).
Sabbatini, P., Lin, J., Levine, A.J. & White, E. Essential role for p53-mediated transcription in E1A-induced apoptosis. Genes Dev. 9, 2184–2192 (1995).
Walker, K.K. & Levine, A.J. Identification of a novel p53 functional domain that is necessary for efficient growth suppression. Proc. Natl Acad. Sci. USA 93, 15335–15340 (1996).
Lee, S., Elenbaas, B., Levine, A. & Griffith, J. p53 and its 14 kDa C-terminal domain recognize primary DNA damage in the form of insertion/deletion mismatches. Cell 81, 1013–1020 (1995).
Mummenbrauer, T. et al. p53 Protein exhibits 3′-to-5′ exonuclease activity. Cell 85, 1089–1099 (1996).
Sturzbecher, H.W., Donzelmann, B., Henning, W., Knippschild, U. & Buchhop, S. p53 is linked directly to homologous recombination processes via RAD51/RecA protein interaction. EMBO J. 15, 1992–2002 (1996).
Lin, J., Chen, J., Elenbaas, B. & Levine, A.J. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 8, 1235–1246 (1994).
O'Gorman, S., Dagenais, N.A., Qian, M. & Marchuk, Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl Acad. Sci. USA 94, 14602–14607 (1997).
Sah, V.P. et al. A subset of p53-deficient embryos exhibit exencephaly. Nature Genet. 10, 175–180 (1995).
Kussie, P.H. et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 274, 948–953 (1996).
Kubbutat, M.H., Jones, S.N. & Vousden, K.H. Regulation of p53 stability by Mdm2. Nature 387, 299–303 (1997).
Haupt, Y., Maya, R., Kazaz, A. & Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 (1997).
Juven-Gershon, T. & Oren, M. Mdm2: the ups and downs. Mol. Med. 5, 71–83 (1999).
McLure, K.G. & Lee, P.W. How p53 binds DNA as a tetramer. EMBO J. 17, 3342–3350 (1998).
Zauberman, A., Barak, Y., Ragimov, N., Levy, N. & Oren, M. Sequence-specific DNA binding by p53: identification of target sites and lack of binding to p53-MDM2 complexes. EMBO J. 12, 2799–2808 (1993).
Kapoor, M., Hamm, R., Yan, W., Taya, Y. & Lozano, G. Cooperative phosphorylation at multiple sites is required to activate p53 in response to UV radiation. Oncogene 19, 358–364 (2000).
Bottger, A. et al. Molecular characterization of the hdm2-p53 interaction. J. Mol. Biol. 269, 744–756 (1997).
Ljungman, M., Zhang, F., Chen, F., Rainbow, A.J. & McKay, B.C. Inhibition of RNA polymerase II as a trigger for the p53 response. Oncogene 18, 583–592 (1999).
Roth, J., Dobbelstein, M., Freedman, D.A., Shenk, T. & Levine, A.J. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 17, 554–564 (1998).
Freedman, D.A. & Levine, A.J. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell. Biol. 18, 7288–7293 (1998).
Stommel, J.M. et al. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 18, 1660–1672 (1999).
de Stanchina, E. et al. E1A signaling to p53 involves the p19(ARF) tumor suppressor. Genes Dev. 12, 2434–2442 (1998).
Zindy, F. et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 12, 2424–2433 (1998).
Khan, S.K., Moritsugu, J. & Wahl, G.M. Differential requirement for p19ARF in the p53-dependent arrest induced by DNA damage, microtubule disruption and ribonucleotide depletion. Proc. Natl Acad. Sci. USA 97, 3266–3271 (2000).
Sherr, C.J. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 12, 2984–2991 (1998).
Kastan, M.B., Onyekwere, O., Sidransky, D., Vogelstein, B. & Craig, R.W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51, 6304–6311 (1991).
Linke, S.P., Clarkin, K.C., Di Leonardo, A., Tsou, A. & Wahl, G.M. A reversible p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev. 10, 934–947 (1996).
Kern, S.E. et al. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science 256, 827–830 (1992).
Lowe, S.W., Schmitt, E.M., Smith, S.W., Osborne, B.A. & Jacks, T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362, 847–849 (1993).
Clarke, A.R. et al. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 362, 849–852 (1993).
Wahl, G.M., Linke, S.P., Paulson, T.G. & Huang, L.C. Maintaining genetic stability through TP53 mediated checkpoint control. Cancer Surv. 29, 183–219 (1997).
Ford, J.M. & Hanawalt, P.C. Li-Fraumeni syndrome fibroblasts homozygous for p53 mutations are deficient in global DNA repair but exhibit normal transcription-coupled repair and enhanced UV resistance. Proc. Natl Acad. Sci. USA 92, 8876–8880 (1995).
Tanaka, H. et al. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature 404, 42–49 (2000).
Wang, X.W. et al. The XPB and XPD DNA helicases are components of the p53-mediated apoptosis pathway. Genes Dev. 10, 1219–1232 (1996).
Tybulewicz, V.L., Crawford, C.E., Jackson, P.K., Bronson, R.T. & Mulligan, R.C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65, 1153–1163 (1991).
Jimenez, G.S. et al. DNA-dependent protein kinase is not required for the p53-dependent response to DNA damage. Nature 400, 81–83 (1999).
Donehower, L.A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992)
Acknowledgements
We thank C. Barlow for help with isolation of mouse thymocytes and advice on measuring apoptosis and tumorigenicity; and M. Vogt and M. Haas for helpful discussions regarding transformation assays. This work was supported by a postdoctoral fellowship from the NIH (G.S.J.), an NSF Graduate Fellowship (J.M.S.) and an ACS International Cancer Research Fellowship from the UICC (M.N.), and by grants from the NIH (G.M.W. and S.O.) and the G. Harold and Leila Y. Mathers Charitable Foundation (G.M.W.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jimenez, G., Nister, M., Stommel, J. et al. A transactivation-deficient mouse model provides insights into Trp53 regulation and function. Nat Genet 26, 37–43 (2000). https://doi.org/10.1038/79152
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/79152
This article is cited by
-
Sensitivity to tumor development by TALEN-mediated Trp53 mutant genes in the susceptible FVB/N mice and the resistance C57BL/6 mice
Laboratory Animal Research (2021)
-
BIM and NOXA are mitochondrial effectors of TAF6δ-driven apoptosis
Cell Death & Disease (2018)
-
Fuse binding protein antagonizes the transcription activity of tumor suppressor protein p53
BMC Cancer (2014)
-
TAF6δ orchestrates an apoptotic transcriptome profile and interacts functionally with p53
BMC Molecular Biology (2010)
-
p53-mediated apoptosis prevents the accumulation of progenitor B cells and B-cell tumors
Cell Death & Differentiation (2010)