Abstract
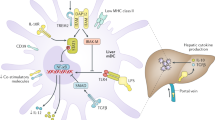
Liver transplantation is a successful treatment for select forms of liver disease and is unrivalled amongst other forms of solid organ transplantation in the ability of the recipient to develop long-term tolerance to the allograft. Much of this success can be attributed to the inherently tolerogenic manner in which antigens are presented in the liver and the presence of specialized regulatory lymphocyte populations. These observations are not universal, however, and a proportion of recipients develop problematic allograft rejection. Anti-allograft responses lead to an influx of effector cells that target hepatic parenchymal cells and induce apoptosis through members of the tumor necrosis factor superfamily. Anti-rejection therapies have traditionally targeted the entire lymphocyte response against the allograft and, whilst these therapies have been efficacious at limiting effector cell responses, they have also had deleterious effects on the regulatory cell populations that are required to promote long-term tolerance. The emergence of newer anti-rejection drugs that have the potential to target the effector response selectively, whilst preserving the much needed tolerogenic responses, has afforded us the opportunity to refine our future immunosuppressant strategies.
Key Points
-
Liver transplantation is an effective treatment for select patients with liver disease, but its success is still hampered by allograft rejection
-
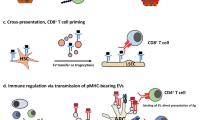
Antigen presentation in the liver is skewed towards tolerance and encourages allograft acceptance
-
Loss of tolerance and a shift towards an anti-allograft effector response leads to sustained targeting and apoptosis of cholangiocytes and hepatocytes through members of the tumor necrosis factor superfamily
-
Regulatory T cells have an important role in allograft tolerance
-
Modern anti-rejection therapies should be tailored to preserve regulatory T-cell function whilst targeting the effector responses
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Barber K et al. (2007) Life expectancy of adult liver allograft recipients in the UK. Gut 56: 279–282
Ormonde DG et al. (1999) Banff schema for grading liver allograft rejection: utility in clinical practice. Liver Transpl Surg 5: 261–268
Perry I and Neuberger J (2005) Immunosuppression: towards a logical approach in liver transplantation. Clin Exp Immunol 139: 2–10
Crispe IN et al. (2006) Cellular and molecular mechanisms of liver tolerance. Immunol Rev 213: 101–118
Adams DH and Afford SC (2005) Effector mechanisms of nonsuppurative destructive cholangitis in graft-versus-host disease and allograft rejection. Semin Liver Dis 25: 281–297
Afford SC et al. (2001) CD40 activation-induced, Fas-dependent apoptosis and NF-kappaB/AP-1 signaling in human intrahepatic biliary epithelial cells. FASEB J 15: 2345–2354
Hubscher SG (2006) Transplantation pathology. Semin Diagn Pathol 23: 170–181
Eksteen B et al. (2007) Immune-mediated liver injury. Semin Liver Dis 27: 351–366
Wood KJ and Sakaguchi S (2003) Regulatory T cells in transplantation tolerance. Nat Rev Immunol 3: 199–210
Morelli AE and Thomson AW (2007) Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol 7: 610–621
Karim M et al. (2005) CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood 105: 4871–4877
Crispe IN (2003) Hepatic T cells and liver tolerance. Nat Rev Immunol 3: 51–62
Bowen DG et al. (2005) Intrahepatic immunity: a tale of two sites. Trends Immunol 26: 512–517
Willberg C et al. (2003) HCV immunology—death and the maiden T cell. Cell Death Differ 10 (Suppl 1): S39–S47
Klugewitz K et al. (2002) Immunomodulatory effects of the liver: deletion of activated CD4+ effector cells and suppression of IFN-gamma-producing cells after intravenous protein immunization. J Immunol 169: 2407–2413
Limmer A et al. (2005) Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol 35: 2970–2981
John B and Crispe IN (2005) TLR-4 regulates CD8+ T cell trapping in the liver. J Immunol 175: 1643–1650
Li W et al. (2001) Il-12 antagonism enhances apoptotic death of T cells within hepatic allografts from Flt3 ligand-treated donors and promotes graft acceptance. J Immunol 166: 5619–5628
Randow F et al. (1995) Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med 181: 1887–1892
Medvedev AE et al. (2002) Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol 169: 5209–5216
Polakos NK et al. (2007) Early intrahepatic accumulation of CD8+ T cells provides a source of effectors for nonhepatic immune responses. J Immunol 179: 201–210
Klugewitz K et al. (2004) The spectrum of lymphoid subsets preferentially recruited into the liver reflects that of resident populations. Immunol Lett 93: 159–162
Gremion C et al. (2004) Cytotoxic T lymphocytes derived from patients with chronic hepatitis C virus infection kill bystander cells via Fas–FasL interaction. J Virol 78: 2152–2157
Hingorani R et al. (2000) CD95/Fas signaling in T lymphocytes induces the cell cycle control protein p21cip-1/WAF-1, which promotes apoptosis. J Immunol 164: 4032–4036
John B et al. (2007) Immune role of hepatic TLR-4 revealed by orthotopic mouse liver transplantation. Hepatology 45: 178–186
Bowen DG et al. (2004) The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest 114: 701–712
Antonysamy MA et al. (1999) Evidence for a role of IL-17 in alloimmunity: a novel IL-17 antagonist promotes heart graft survival. Transplant Proc 31: 93
Denning TL et al. (2007) Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol 8: 1086–1094
Shortman K and Liu YJ (2002) Mouse and human dendritic cell subtypes. Nat Rev Immunol 2: 151–161
Khanna A et al. (2000) Effects of liver-derived dendritic cell progenitors on Th1- and Th2-like cytokine responses in vitro and in vivo. J Immunol 164: 1346–1354
Lai WK et al. (2007) Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. J Hepatol 47: 338–347
Goddard S et al. (2004) Interleukin-10 secretion differentiates dendritic cells from human liver and skin. Am J Pathol 164: 511–519
Lai WK et al. (2007) Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. J Hepatol 47: 338–347
Kubo T et al. (2004) Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol 173: 7249–7258
Kuwana M (2002) Induction of anergic and regulatory T cells by plasmacytoid dendritic cells and other dendritic cell subsets. Hum Immunol 63: 1156–1163
Mazzoni A et al. (2003) Cutting edge: histamine inhibits IFN-alpha release from plasmacytoid dendritic cells. J Immunol 170: 2269–2273
Ito T et al. (2007) Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med 204: 105–115
Tsung A et al. (2005) Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol 175: 7661–7668
Tsung A et al. (2007) Increasing numbers of hepatic dendritic cells promote HMGB1-mediated ischemia-reperfusion injury. J Leukoc Biol 81: 119–128
Lalor PF and Adams DH (2002) The liver : a model of organ-specific lymphocyte recruitment. Expert Rev Mol Med 4: 1–16
Thomson AW et al. (1995) Identification of donor-derived dendritic cell progenitors in bone marrow of spontaneously tolerant liver allograft recipients. Transplantation 60: 1555–1559
Bishop GA et al. (1996) Tolerance to rat liver allografts. III. Donor cell migration and tolerance-associated cytokine production in peripheral lymphoid tissues. J Immunol 156: 4925–4931
Gould DS and Auchincloss H Jr (1999) Direct and indirect recognition: the role of MHC antigens in graft rejection. Immunol Today 20: 77–82
Herrera OB et al. (2004) A novel pathway of alloantigen presentation by dendritic cells. J Immunol 173: 4828–4837
Thery C et al. (2002) Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 3: 1156–1162
Abe M and Thomson AW (2003) Influence of immunosuppressive drugs on dendritic cells. Transpl Immunol 11: 357–365
Turnquist HR et al. (2007) Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol 178: 7018–7031
Lu L et al. (1997) Blockade of the CD40-CD40 ligand pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival. Transplantation 64: 1808–1815
Lan YY et al. (2006) “Alternatively activated” dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol 177: 5868–5877
Mellor AL et al. (2004) Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol 16: 1391–1401
Garrovillo M et al. (1999) Indirect allorecognition in acquired thymic tolerance: induction of donor-specific tolerance to rat cardiac allografts by allopeptide-pulsed host dendritic cells. Transplantation 68: 1827–1834
Ochando JC et al. (2006) Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol 7: 652–662
Sakaguchi S (2004) Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 22: 531–562
Groux H et al. (1997) A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389: 737–742
Weiner HL (2001) Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect 3: 947–954
Sakaguchi S et al. (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 212: 8–27
Sakaguchi S (2005) Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6: 345–352
Seddiki N et al. (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203: 1693–1700
Ziegler SF (2005) FOXP3: of mice and men. Annu Rev Immunol 24: 209–226
Bennett CL and Ochs HD (2001) IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr 13: 533–538
Fontenot JD et al. (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336
de la Rosa M et al. (2004) Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol 34: 2480–2488
Coombes JL et al. (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204: 1757–1764
Lehmann J et al. (2002) Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25− regulatory T cells. Proc Natl Acad Sci USA 99: 13031–13036
Ruprecht CR et al. (2005) Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med 201: 1793–1803
Levings MK and Roncarolo MG (2005) Phenotypic and functional differences between human CD4+CD25+ and type 1 regulatory T cells. Curr Top Microbiol Immunol 293: 303–326
Carrier Y et al. (2007) Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol 178: 179–185
Roncarolo MG and Battaglia M (2007) Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol 7: 585–598
Lan RY et al. (2006) Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology 43: 729–737
Rushbrook SM et al. (2005) Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol 79: 7852–7859
Unitt E et al. (2005) Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology 41: 722–730
Agarwal K et al. (2000) Cytotoxic T lymphocyte antigen-4 (CTLA-4) gene polymorphisms and susceptibility to type 1 autoimmune hepatitis. Hepatology 31: 49–53
Longhi MS et al. (2006) Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol 176: 4484–4491
Li W et al. (2006) The role of Foxp3+ regulatory T cells in liver transplant tolerance. Transplant Proc 38: 3205–3206
Jiang X et al. (2006) The importance of CD25(+)CD4(+) regulatory T cells in mouse hepatic allograft tolerance. Liver Transpl 12: 1112–1118
Li W et al. (2006) Anti-CD25 mAb administration prevents spontaneous liver transplant tolerance. Transplant Proc 38: 3207–3208
Xia G et al. (2006) Targeting acute allograft rejection by immunotherapy with ex vivo-expanded natural CD4+ CD25+ regulatory T cells. Transplantation 82: 1749–1755
Pu LY et al. (2007) Adoptive transfusion of ex vivo donor alloantigen-stimulated CD4(+)CD25(+) regulatory T cells ameliorates rejection of DA-to-Lewis rat liver transplantation. Surgery 142: 67–73
Demirkiran A et al. (2007) Allosuppressive donor CD4+CD25+ regulatory T cells detach from the graft and circulate in recipients after liver transplantation. J Immunol 178: 6066–6072
Koshiba T et al. (2007) Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transpl Immunol 17: 94–97
Demirkiran A et al. (2006) Low circulating regulatory T-cell levels after acute rejection in liver transplantation. Liver Transpl 12: 277–284
VanBuskirk AM et al. (2000) Human allograft acceptance is associated with immune regulation. J Clin Invest 106: 145–155
Steger U et al. (2006) CD25+CD4+ regulatory T cells develop in mice not only during spontaneous acceptance of liver allografts but also after acute allograft rejection. Transplantation 82: 1202–1209
Goddard S and Adams DH (2002) Methylprednisolone therapy for acute rejection: too much of a good thing. Liver Transpl 8: 535–536
Calne R (1996) WOFIE hypothesis: some thoughts on an approach toward allograft tolerance. Transplant Proc 28: 1152
Wang C et al. (2001) A short course of methylprednisolone immunosuppression inhibits both rejection and spontaneous acceptance of rat liver allografts. Transplantation 72: 44–51
Dresske B et al. (2006) WOFIE stimulates regulatory T cells: a 2-year follow-up of renal transplant recipients. Transplantation 81: 1549–1557
Karagiannidis C et al. (2004) Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol 114: 1425–1433
Chen X et al. (2006) Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol 36: 2139–2149
Barrat FJ et al. (2002) In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med 195: 603–616
Emmer PM et al. (2006) Dendritic cells activated by lipopolysaccharide after dexamethasone treatment induce donor-specific allograft hyporesponsiveness. Transplantation 81: 1451–1459
Gregori S et al. (2001) Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol 167: 1945–1953
Pedersen AE et al. (2004) Induction of regulatory dendritic cells by dexamethasone and 1alpha,25-Dihydroxyvitamin D(3). Immunol Lett 91: 63–69
Mueller XM (2004) Drug immunosuppression therapy for adult heart transplantation. Part 1: immune response to allograft and mechanism of action of immunosuppressants. Ann Thorac Surg 77: 354–362
Segundo DS et al. (2006) Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation 82: 550–557
Keever-Taylor CA et al. (2007) Rapamycin enriches for CD4(+) CD25(+) CD27(+) Foxp3(+) regulatory T cells in ex vivo-expanded CD25-enriched products from healthy donors and patients with multiple sclerosis. Cytotherapy 9: 144–157
Strauss G et al. (2002) Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clin Exp Immunol 128: 255–266
Strauss L et al. (2007) Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol 178: 320–329
Coenen JJ et al. (2007) Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant 39: 537–545
Battaglia M et al. (2006) Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes 55: 40–49
Hirose R et al. (2000) Experience with daclizumab in liver transplantation: renal transplant dosing without calcineurin inhibitors is insufficient to prevent acute rejection in liver transplantation. Transplantation 69: 307–311
Calmus Y et al. (2002) Immunoprophylaxis with basiliximab, a chimeric anti-interleukin-2 receptor monoclonal antibody, in combination with azathioprine-containing triple therapy in liver transplant recipients. Liver Transpl 8: 123–131
Baan CC et al. (2005) Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation 80: 110–117
Kreijveld E et al. (2007) Following anti-CD25 treatment, a functional CD4+CD25+ regulatory T-cell pool is present in renal transplant recipients. Am J Transplant 7: 249–255
Vlad G et al. (2007) Anti-CD25 treatment and FOXP3-positive regulatory T cells in heart transplantation. Transpl Immunol 18: 13–21
Tryphonopoulos P et al. (2005) The impact of Campath 1H induction in adult liver allotransplantation. Transplant Proc 37: 1203–1204
Kirsch BM et al. (2006) Alemtuzumab (Campath-1H) induction therapy and dendritic cells: impact on peripheral dendritic cell repertoire in renal allograft recipients. Transpl Immunol 16: 254–257
Hoffmann P et al. (2004) Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood 104: 895–903
Fujio K et al. (2007) T cell receptor gene therapy for autoimmune diseases. Ann N Y Acad Sci 1110: 222–232
Hubscher SG (2006) Transplantation pathology. Semin Diagn Pathol 23: 170–181
Horoldt BS et al. (2006) Does the Banff rejection activity index predict outcome in patients with early acute cellular rejection following liver transplantation. Liver Transpl 12: 1144–1151
Demetris AJ et al. (2006) Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology 44: 489–501
Demetris A et al. (2000) Update of the International Banff Schema for Liver Allograft Rejection: Working Recommendations for the Histopathologic Staging and Reporting of Chronic Rejection. Hepatology 31: 792–799
Acknowledgements
The help and constructive criticism of Professor Kathryn Wood in the preparation of the manuscript is gratefully acknowledged. We are also indebted to Professor David Adams and Dr Simon Afford at the University of Birmingham whose work in this field has formed the basis of many of the concepts discussed in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
James M Neuberger has received speaker support from Hoffmann-La Roche, Novartis and Astellas, and is a consultant for Bristol-Myers. Bertus Eksteen declared no competing interests.
Rights and permissions
About this article
Cite this article
Eksteen, B., Neuberger, J. Mechanisms of Disease: the evolving understanding of liver allograft rejection. Nat Rev Gastroenterol Hepatol 5, 209–219 (2008). https://doi.org/10.1038/ncpgasthep1070
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpgasthep1070