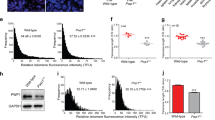
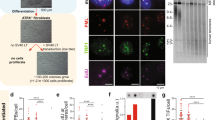
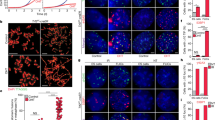
Abstract
The tumour suppressor p53 activates Puma-dependent apoptosis and p21-dependent cell-cycle arrest in response to DNA damage. Deletion of p21 improved stem-cell function and organ maintenance in progeroid mice with dysfunctional telomeres, but the function of Puma has not been investigated in this context. Here we show that deletion of Puma improves stem- and progenitor-cell function, organ maintenance and lifespan of telomere-dysfunctional mice. Puma deletion impairs the clearance of stem and progenitor cells that have accumulated DNA damage as a consequence of critically short telomeres. However, further accumulation of DNA damage in these rescued progenitor cells leads to increasing activation of p21. RNA interference experiments show that upregulation of p21 limits proliferation and evolution of chromosomal imbalances of Puma-deficient stem and progenitor cells with dysfunctional telomeres. These results provide experimental evidence that p53-dependent apoptosis and cell-cycle arrest act in cooperating checkpoints limiting tissue maintenance and evolution of chromosomal instability at stem- and progenitor-cell levels in response to telomere dysfunction. Selective inhibition of Puma-dependent apoptosis can result in temporary improvements in maintenance of telomere-dysfunctional organs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
28 January 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41556-021-00633-w
References
Kujoth, G. C. et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484 (2005).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Hasty, P., Campisi, J., Hoeijmakers, J., van Steeg, H. & Vijg, J. Aging and genome maintenance: lessons from the mouse? Science 299, 1355–1359 (2003).
Migliaccio, E. et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402, 309–313 (1999).
Hamann, A., Brust, D. & Osiewacz, H. D. Apoptosis pathways in fungal growth, development and ageing. Trends Microbiol. 16, 276–283 (2008).
Perez, G. I. et al. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat. Genet. 21, 200–203 (1999).
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97, 527–538 (1999).
Begus-Nahrmann, Y. et al. p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nat. Genet. 41, 1138–1143 (2009).
Erlacher, M. et al. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 106, 4131–4138 (2005).
Jeffers, J. R. et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4, 321–328 (2003).
Villunger, A. et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302, 1036–1038 (2003).
Wu, W. S. et al. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 123, 641–653 (2005).
Michalak, E. M., Villunger, A., Adams, J. M. & Strasser, A. In several cell types tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ. 15, 1019–1029 (2008).
Liu, D. et al. Puma is required for p53-induced depletion of adult stem cells. Nat. Cell Biol. 12, 993–998 (2010).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Choudhury, A. R. et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat. Genet. 39, 99–105 (2007).
Schaetzlein, S. et al. Exonuclease-1 deletion impairs DNA damage signaling and prolongs lifespan of telomere-dysfunctional mice. Cell 130, 863–877 (2007).
Wong, K. K. et al. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421, 643–648 (2003).
van der Flier, L. G. et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136, 903–912 (2009).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Kirk, K. E., Harmon, B. P., Reichardt, I. K., Sedat, J. W. & Blackburn, E. H. Block in anaphase chromosome separation caused by a telomerase template mutation. Science 275, 1478–1481 (1997).
Rudolph, K. L., Millard, M., Bosenberg, M. W. & DePinho, R. A. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat. Genet. 28, 155–159 (2001).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Cheng, H. & Leblond, C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 141, 537–561 (1974).
Potten, C. S. et al. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 71, 28–41 (2003).
Hastie, N. D. et al. Telomere reduction in human colorectal carcinoma and with ageing. Nature 346, 866–868 (1990).
Jiang, H. et al. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc. Natl Acad. Sci. USA 105, 11299–11304 (2008).
Aggarwal, S. & Gupta, S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J. Immunol. 160, 1627–1637 (1998).
Ciccocioppo, R. et al. Small bowel enterocyte apoptosis and proliferation are increased in the elderly. Gerontology 48, 204–208 (2002).
Hashimoto, S., Ochs, R. L., Komiya, S. & Lotz, M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 41, 1632–1638 (1998).
Mustata, G. et al. Development of small-molecule PUMA inhibitors for mitigating radiation-induced cell death. Curr. Top. Med. Chem. 11, 281–290.
Wiemann, S. U. et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 16, 935–942 (2002).
Armanios, M. Y. et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 356, 1317–1326 (2007).
Ohyashiki, J. H. et al. Telomere shortening associated with disease evolution patterns in myelodysplastic syndromes. Cancer Res. 54, 3557–3560 (1994).
Herrera, E. et al. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 18, 2950–2960 (1999).
Geigl, J. B. & Speicher, M. R. Single-cell isolation from cell suspensions and whole genome amplification from single cells to provide templates for CGH analysis. Nat. Protoc. 2, 3173–3184 (2007).
van Beers, E. H. et al. A multiplex PCR predictor for aCGH success of FFPE samples. Br. J. Cancer 94, 333–337 (2006).
Acknowledgements
We thank G. Zambetti for providing P u m a−/− mice, C. Kuo for the R-spondin construct and H. Clevers for discussions. The Deutsche Forschungsgemeinschaft (Klinische Forschergruppe 142 & 167 and Ru745/10) and the European Union (GENINCA) supported this work.
Author information
Authors and Affiliations
Contributions
T.S., Z.S., Y.M., K.N., Y.B-N., M.D.B. and Z.J. carried out, designed and analysed experiments; T.S., A.L., Y.B-N., M.M. and M.R.S. carried out and analysed aCGH experiments; F.S. and B.L. carried out microdissection; Z.S. and L.M.G. generated mouse crosses; K.L.R. and T.S. wrote the manuscript; K.L.R. conceived the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1774 kb)
Supplementary Tables 1–3
Supplementary Information (XLS 38 kb)
Rights and permissions
About this article
Cite this article
Sperka, T., Song, Z., Morita, Y. et al. Puma and p21 represent cooperating checkpoints limiting self-renewal and chromosomal instability of somatic stem cells in response to telomere dysfunction. Nat Cell Biol 14, 73–79 (2012). https://doi.org/10.1038/ncb2388
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2388
This article is cited by
-
Bax deficiency extends the survival of Ku70 knockout mice that develop lung and heart diseases
Cell Death & Disease (2015)
-
The Role of Stem Cell Genomic Instability in Aging
Current Stem Cell Reports (2015)
-
Chronic inflammation induces telomere dysfunction and accelerates ageing in mice
Nature Communications (2014)
-
Telomere stability—Wnt it or lose it
EMBO reports (2013)
-
Splice variants DNMT3B4 and DNMT3B7 overexpression inhibit cell proliferation in 293A cell line
In Vitro Cellular & Developmental Biology - Animal (2013)